Effects of a novel mobile health intervention compared to a multi-component behaviour changing program on body mass index, physical capacities and stress parameters in adolescents with obesity: a randomized controlled trial
- PMID: 34243738
- PMCID: PMC8266630
- DOI: 10.1186/s12887-021-02781-2
Effects of a novel mobile health intervention compared to a multi-component behaviour changing program on body mass index, physical capacities and stress parameters in adolescents with obesity: a randomized controlled trial
Abstract
Background: Less than 2% of overweight children and adolescents in Switzerland can participate in multi-component behaviour changing interventions (BCI), due to costs and lack of time. Stress often hinders positive health outcomes in youth with obesity. Digital health interventions, with fewer on-site visits, promise health care access in remote regions; however, evidence for their effectiveness is scarce.
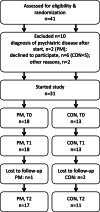
Methods: This randomized controlled not blinded trial (1:1) was conducted in a childhood obesity center in Switzerland. Forty-one youth aged 10-18 years with body mass index (BMI) > P.90 with risk factors or co-morbidities or BMI > P.97 were recruited. During 5.5 months, the PathMate2 group (PM) received daily conversational agent counselling via mobile app, combined with standardized counselling (4 on-site visits). Controls (CON) participated in a BCI (7 on-site visits). We compared the outcomes of both groups after 5.5 (T1) and 12 (T2) months. Primary outcome was reduction in BMI-SDS (BMI standard deviation score: BMI adjusted for age and sex). Secondary outcomes were changes in body fat and muscle mass (bioelectrical impedance analysis), waist-to-height ratio, physical capacities (modified Dordel-Koch-Test), blood pressure and pulse. Additionally, we hypothesized that less stressed children would lose more weight. Thus, children performed biofeedback relaxation exercises while stress parameters (plasma cortisol, stress questionnaires) were evaluated.
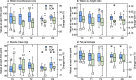
Results: At intervention start median BMI-SDS of all patients (18 PM, 13 CON) was 2.61 (obesity > + 2SD). BMI-SDS decreased significantly in CON at T1, but not at T2, and did not decrease in PM during the study. Muscle mass, strength and agility improved significantly in both groups at T2; only PM reduced significantly their body fat at T1 and T2. Average daily PM app usage rate was 71.5%. Cortisol serum levels decreased significantly after biofeedback but with no association between stress parameters and BMI-SDS. No side effects were observed.
Conclusions: Equally to BCI, PathMate2 intervention resulted in significant and lasting improvements of physical capacities and body composition, but not in sustained BMI-SDS decrease. This youth-appealing mobile health intervention provides an interesting approach for youth with obesity who have limited access to health care. Biofeedback reduces acute stress and could be an innovative adjunct to usual care.
Keywords: Adolescents; Biofeedback; Conversational agent; Digital health interventions; Fitness; Obesity; Stress.
© 2021. The Author(s).
Conflict of interest statement
CHIS and TK are affiliated with the Centre for Digital Health Interventions (
The other authors declare that they have no competing interests.
Figures




References
-
- Ells LJ, Rees K, Brown T, Mead E, Al-Khudairy L, Azevedo L. Interventions for treating children and adolescents with overweight and obesity: an overview of Cochrane reviews. Int J Obes (Lond). 2018;42(11):1823–1833; doi: 10.1038/s41366-018-0230-y. Epub 2018 Oct 9. Erratum in: Int J Obes (Lond). 2019 Apr 2. - PubMed
-
- Sempach R, Farpour-Lambert NJ, L’Allemand D, Laimbacher J. Therapie des adipösen Kindes und Jugendlichen: Vorschläge für multiprofessionelle Therapieprogramme. (Therapy of the obese child and adolescent: Suggestions for multi-professional therapy programs. Article in German.) Paediatrica. 2007;18(2):33–39.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

