Tocilizumab therapy in SARS-CoV-2 pneumonia: A matched retrospective cohort analysis
- PMID: 34243954
- PMCID: PMC8260823
- DOI: 10.1016/j.medcli.2021.06.014
Tocilizumab therapy in SARS-CoV-2 pneumonia: A matched retrospective cohort analysis
Abstract
Background: The effect of immunomodulatory therapy with tocilizumab for coronavirus disease 2019 (COVID-19) in real-life clinical practice remains controversial.
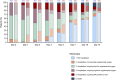
Methods: Single-center retrospective matched cohort analysis including 47 consecutive patients treated with intravenous tocilizumab for severe COVID-19 pneumonia ("TCZ group"), matched by age, comorbidities, time from symptoms onset and baseline SpO2/FiO2 ratio with 47 patients receiving standard of care alone ("SoC group").
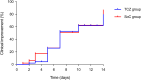
Results: There were no significant differences between the TCZ and SoC groups in the rate of clinical improvement (hospital discharge and/or a decrease of ≥2 points on a six-point ordinal scale) by day 7 (51.1% [24/47] versus 48.9% [23/47]; P-value=1.000). No differences were observed at day 14 in terms of clinical improvement (72.3% versus 76.6%; P-value=0.791), all-cause mortality (10.6% versus 12.8%; P-value=1.000), and the composite of invasive mechanical ventilation and/or death (25.5% versus 23.4%; P-value=1.000) either. Patients in the TCZ group had a more rapid normalization of C-reactive protein levels.
Conclusions: No apparent benefit was observed in patients with severe COVID-19 treated with tocilizumab as compared to a matched retrospective cohort.
Antecedentes: El efecto del tratamiento inmunomodulador con tocilizumab en la COVID-19 sigue siendo controvertido.
Métodos: Estudio unicéntrico de cohortes retrospectivas pareadas que incluyó a 47 pacientes con COVID-19 grave tratados con tocilizumab intravenoso («grupo TCZ»), emparejados por edad, comorbilidades mayores, evolución de síntomas y cociente SpO2/FiO2 basal con 47 pacientes que recibieron tratamiento estándar únicamente («grupo SoC»).
Resultados: No observamos diferencias significativas entre los grupos de TCZ y SoC en la tasa de mejoría clínica (alta hospitalaria y/o descenso de ≥ 2 puntos en una escala ordinal de 6 puntos) al día 7 (51,1% [24/47] vs. 48,9% [23/47]; P = 1,000). Tampoco hubo diferencias al día 14 en las tasas de mejoría clínica (72,3% vs. 76,6%; P = 0,791), mortalidad (10,6% vs. 12,8%; P = 1,000) o en el compuesto de ventilación mecánica invasiva y/o muerte (25,5% vs. 23,4%; P = 1,000). Los pacientes en el grupo de TCZ presentaron una normalización más rápida de la proteína C reactiva.
Conclusiones: Respecto a una cohorte retrospectiva pareada, no detectamos un beneficio asociado al tratamiento con tocilizumab en pacientes con neumonía por COVID-19.
Keywords: COVID-19; Estudio de cohortes pareadas; Evolución; Immunomodulatory therapy; Matched cohort study; Outcome; Tocilizumab; Tratamiento inmunomodulador.
Copyright © 2021 Elsevier España, S.L.U. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

