The fluid shear stress sensor TRPM7 regulates tumor cell intravasation
- PMID: 34244134
- PMCID: PMC8270498
- DOI: 10.1126/sciadv.abh3457
The fluid shear stress sensor TRPM7 regulates tumor cell intravasation
Abstract
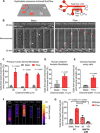
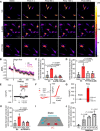
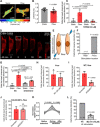
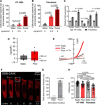
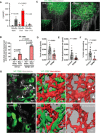
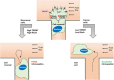
Tumor cell intravasation preferentially occurs in regions of low fluid shear because high shear is detrimental to tumor cells. Here, we describe a molecular mechanism by which cells avoid high shear during intravasation. The transition from migration to intravasation was modeled using a microfluidic device where cells migrating inside longitudinal tissue-like microchannels encounter an orthogonal channel in which fluid flow induces physiological shear stresses. This approach was complemented with intravital microscopy, patch-clamp, and signal transduction imaging techniques. Fluid shear-induced activation of the transient receptor potential melastatin 7 (TRPM7) channel promotes extracellular calcium influx, which then activates RhoA/myosin-II and calmodulin/IQGAP1/Cdc42 pathways to coordinate reversal of migration direction, thereby avoiding shear stress. Cells displaying higher shear sensitivity due to higher TRPM7 activity levels intravasate less efficiently and establish less invasive metastatic lesions. This study provides a mechanistic interpretation for the role of shear stress and its sensor, TRPM7, in tumor cell intravasation.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

