Temporal compartmentalization of viral infection in bacterial cells
- PMID: 34244425
- PMCID: PMC8285916
- DOI: 10.1073/pnas.2018297118
Temporal compartmentalization of viral infection in bacterial cells
Abstract
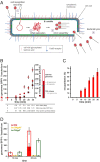
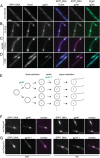
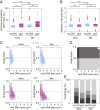
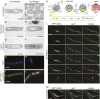
Virus infection causes major rearrangements in the subcellular architecture of eukaryotes, but its impact in prokaryotic cells was much less characterized. Here, we show that infection of the bacterium Bacillus subtilis by bacteriophage SPP1 leads to a hijacking of host replication proteins to assemble hybrid viral-bacterial replisomes for SPP1 genome replication. Their biosynthetic activity doubles the cell total DNA content within 15 min. Replisomes operate at several independent locations within a single viral DNA focus positioned asymmetrically in the cell. This large nucleoprotein complex is a self-contained compartment whose boundaries are delimited neither by a membrane nor by a protein cage. Later during infection, SPP1 procapsids localize at the periphery of the viral DNA compartment for genome packaging. The resulting DNA-filled capsids do not remain associated to the DNA transactions compartment. They bind to phage tails to build infectious particles that are stored in warehouse compartments spatially independent from the viral DNA. Free SPP1 structural proteins are recruited to the dynamic phage-induced compartments following an order that recapitulates the viral particle assembly pathway. These findings show that bacteriophages restructure the crowded host cytoplasm to confine at different cellular locations the sequential processes that are essential for their multiplication.
Keywords: bacterial cell compartmentalization; bacteriophage; phage DNA replication; virus assembly; virus infection.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

