1,2-Difunctionalized bicyclo[1.1.1]pentanes: Long-sought-after mimetics for ortho/ meta-substituted arenes
- PMID: 34244445
- PMCID: PMC8285974
- DOI: 10.1073/pnas.2108881118
1,2-Difunctionalized bicyclo[1.1.1]pentanes: Long-sought-after mimetics for ortho/ meta-substituted arenes
Abstract
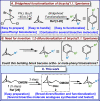
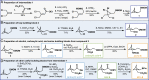
The development of a versatile platform for the synthesis of 1,2-difunctionalized bicyclo[1.1.1]pentanes to potentially mimic ortho/meta-substituted arenes is described. The syntheses of useful building blocks bearing alcohol, amine, and carboxylic acid functional handles have been achieved from a simple common intermediate. Several ortho- and meta-substituted benzene analogs, as well as simple molecular matched pairs, have also been prepared using this platform. The results of in-depth ADME (absorption, distribution, metabolism, and excretion) investigations of these systems are presented, as well as computational studies which validate the ortho- or meta-character of these bioisosteres.
Keywords: bioisosteres; medicinal chemistry; synthesis.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Lovering F., Bikker J., Humblet C., Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009). - PubMed
-
- Lovering F., Escape from flatland 2: Complexity and promiscuity. MedChemComm 4, 515–519 (2013).
-
- Blakemore D. C., et al. ., Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018). - PubMed
-
- Kirichok A. A., Shton I., Kliachyna M., Pishel I., Mykhailiuk P. K., 1-substituted 2-azaspiro[3.3]heptanes: Overlooked motifs for drug discovery. Angew. Chem. Int. Ed. Engl. 56, 8865–8869 (2017). - PubMed
-
- Kirichok A. A., et al. ., Synthesis of multifunctional spirocyclic azetidines and their application in drug discovery. Chemistry 24, 5444–5449 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

