Teprotumumab for the treatment of chronic thyroid eye disease
- PMID: 34244669
- PMCID: PMC9307784
- DOI: 10.1038/s41433-021-01593-z
Teprotumumab for the treatment of chronic thyroid eye disease
Abstract
Background: Teprotumumab, a novel IGF-1R antibody was recently shown to significantly reduce the signs of active Thyroid eye disease (TED). The current study reviews its efficacy in chronic TED.
Methods: In this retrospective review, consecutive patients with chronic stable TED (>2 years), who had received ≥3 infusions of teprotumumab were included. All patients had measurements of proptosis, and calculation of the CAS and diplopia scores before and after therapy. Five-point strabismus scores were also calculated. Patients who had imaging within 4 months prior to therapy and 6 weeks post therapy underwent orbital 3D volumetric analysis.
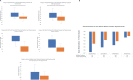
Results: Thirty-one patients met the inclusion criteria. The mean (SD) duration of TED was 81 months (56) and the mean (SD) number of infusions received by each patient was 7 (2). Mean (SD) reduction in proptosis for each study orbit was 3.5 mm (0.4) and 3 mm (0.3) for the fellow orbit. The CAS response was 90% for the study orbit and 87% for the fellow orbit. Of the 15 patients who had diplopia at baseline, 67% had a clinically significant response, while 47% had complete resolution following treatment. Following teprotumumab, mean (SD) reduction of muscle tissue was 2011 mm3 (1847) in the study orbit and 1620 mm3 (1759) in the fellow orbit. The mean (SD) reduction of fat volume was 2101 mm3 (1681) in the study orbit and 1370 mm3 (1181) in the fellow orbit.
Conclusion: Teprotumumab significantly reduces proptosis, inflammation, diplopia, strabismus and orbital soft tissue volume in patients with chronic TED.
© 2021. The Author(s).
Conflict of interest statement
RD—Consultant Horizon Therapeutics, Immunovant Corporation, Veridian Corporation. KC—Consultant Horizon Therapeutics, Viridian Pharmaceuticals and 3T Ophthalmics. AH-Consultant Horizon Therapeutics, Osmotica Pharmaceuticals. AK—Consultant Horizon Therapeutics, Osmotica Pharmaceuticals, Immunovant Corporation, Axogen Corporation. Horizon Therapeutics funded the publication fees after the article was accepted for publication.
Figures



References
-
- Ugradar S, Rootman DB. Orbital fat expansion in thyroid eye disease is related to age. Eur. J. Ophthalmol. 2020;30. - PubMed
-
- Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin. Sci. 1945;5:177–94. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

