Efficacy and Safety of Ticagrelor and Aspirin in Patients With Moderate Ischemic Stroke: An Exploratory Analysis of the THALES Randomized Clinical Trial
- PMID: 34244703
- PMCID: PMC8430457
- DOI: 10.1001/jamaneurol.2021.2440
Efficacy and Safety of Ticagrelor and Aspirin in Patients With Moderate Ischemic Stroke: An Exploratory Analysis of the THALES Randomized Clinical Trial
Abstract
Importance: Prior trials of dual antiplatelet therapy excluded patients with moderate ischemic stroke. These patients were included in the Acute Stroke or Transient Ischaemic Attack Treated With Ticagrelor and ASA for Prevention of Stroke and Death (THALES) trial, but results have not been reported separately, raising concerns about safety and efficacy in this subgroup.
Objective: To evaluate the efficacy and safety of ticagrelor plus aspirin in patients with moderate ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score of 4 to 5).
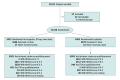
Design, setting, and participants: The THALES trial was a randomized trial conducted at 414 hospitals in 28 countries in January 2018 and December 2019. This exploratory analysis compared patients with moderate stroke (baseline NIHSS score of 4 to 5) with patients with less severe stroke (NIHSS score of 0 to 3). A total of 9983 patients with stroke were included in the present analysis, after excluding 2 patients with NIHSS scores greater than 5 and 1031 patients with transient ischemic attack. Data were analyzed from March to April 2021.
Interventions: Ticagrelor (180-mg loading dose on day 1 followed by 90 mg twice daily on days 2 to 30) or placebo within 24 hours after symptom onset. All patients received aspirin, 300 to 325 mg, on day 1 followed by aspirin, 75 to 100 mg, daily on days 2 to 30. Patients were observed for 30 additional days.
Main outcomes and measures: The primary outcome was time to stroke or death within 30 days. The primary safety outcome was time to severe bleeding.
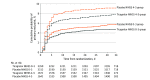
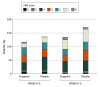
Results: In total, 3312 patients presented with moderate stroke and 6671 presented with less severe stroke. Of those in the moderate stroke group, 1293 (39.0%) were female, and the mean (SD) age was 64.5 (10.8) years; of those in the less severe stroke group, 2518 (37.7%) were female, and the mean (SD) age was 64.8 (11.2) years. The observed primary outcome event rate in patients with moderate stroke was 7.6% (129 of 1671) for those in the ticagrelor group and 9.1% (150 of 1641) for those in the placebo group (hazard ratio, 0.84; 95% CI, 0.66-1.06); the primary outcome event rate in patients with less severe stroke was 4.7% (158 of 3359) for those in the ticagrelor group and 5.7% (190 of 3312) for those in the placebo group (hazard ratio, 0.82; 95% CI, 0.66-1.01) (P for interaction = .88). Severe bleeding occurred in 8 patients (0.5%) in the ticagrelor group and in 4 patients (0.2%) in the placebo group in those with moderate stroke compared with 16 patients (0.5%) and 3 patients (0.1%), respectively, with less severe stroke (P for interaction = .26).
Conclusions and relevance: In this study, patients with a moderate ischemic stroke had consistent benefit from ticagrelor plus aspirin vs aspirin alone compared with patients with less severe ischemic stroke, with no further increase in the risk of intracranial bleeding or other severe bleeding events.
Trial registration: ClinicalTrials.gov Identifier: NCT03354429.
Conflict of interest statement
Figures
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211 - DOI - PubMed
-
- Liu L, Chen W, Zhou H, et al. ; Chinese Stroke Association Stroke Council Guideline Writing Committee . Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. 2020;5(2):159-176. doi:10.1136/svn-2020-000378 - DOI - PMC - PubMed
-
- Dawson J, Merwick Á, Webb A, Dennis M, Ferrari J, Fonseca AC; European Stroke Organisation . European Stroke Organisation expedited recommendation for the use of short-term dual antiplatelet therapy early after minor stroke and high-risk TIA. Eur Stroke J. Published online March 11, 2021. doi:10.1177/23969873211000877 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

