Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model
- PMID: 34244768
- PMCID: PMC8408768
- DOI: 10.1093/infdis/jiab361
Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model
Abstract
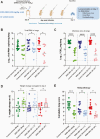
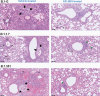
The emergence of SARS-CoV-2 variants of concern (VoCs) has exacerbated the COVID-19 pandemic. Currently available monoclonal antibodies and vaccines appear to have reduced efficacy against some of these VoCs. Antivirals targeting conserved proteins of SARS-CoV-2 are unlikely to be affected by mutations arising in VoCs and should therefore be effective against emerging variants. We here investigate the efficacy of molnupiravir, currently in phase 2 clinical trials, in hamsters infected with Wuhan strain or B.1.1.7 and B.1.351 variants. Molnupiravir proved to be effective against infections with each of the variants and therefore may have potential combating current and future emerging VoCs.
Keywords: B.1.351; SARS-CoV-2; VoC; antivirals; coronavirus; hamsters; molnupiravir.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Wise J. Covid-19: the E484K mutation and the risks it poses. BMJ 2021; 372:n359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

