European clinical guidelines for Tourette syndrome and other tic disorders: summary statement
- PMID: 34244849
- PMCID: PMC8940881
- DOI: 10.1007/s00787-021-01832-4
European clinical guidelines for Tourette syndrome and other tic disorders: summary statement
Abstract
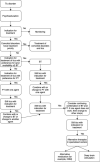
In 2011 a working group of the European Society for the Study of Tourette syndrome (ESSTS) developed the first European Guidelines for Tourette syndrome (TS) published in the ECAP journal. After a decade ESSTS now presents updated guidelines, divided into four sections: Part I: assessment, Part II: psychological interventions, Part III: pharmacological treatment and Part IV: deep brain stimulation (DBS). In this paper, we summarise new developments described in the guidelines with respect to assessment and treatment of tics. Further, summary findings from a recent survey conducted amongst TS experts on these same topics are presented, as well as the first European patient representative statement on research. Finally, an updated decision tree is introduced providing a practical algorithm for the treatment of patients with TS. Interestingly, in the last decade there has been a significant shift in assessment and treatment of tics, with more emphasis on non-pharmacological treatments.
Keywords: Classification; Comorbidities; Guidelines; Tics; Tourette syndrome; Treatment.
© 2021. The Author(s).
Conflict of interest statement
KMV has received financial or material research support from EU (FP7-HEALTH-2011 No. 278367, FP7-PEOPLE-2012-ITN No. 316978) DFG: GZ MU 1527/3-1 and GZ MU 1527/3-2, BMBF: 01KG1421, National Institute of Mental Health (NIMH), Tourette Gesellschaft Deutschland e.V. Else-Kröner-Fresenius-Stiftung, GW pharmaceuticals, Almirall Hermal GmbH, Abide Therapeutics, and Therapix Biosiences. She has received consultant's honoraria from Abide Therapeutics, Boehringer Ingelheim International GmbH, Bionorica Ethics GmbH, CannaMedical Pharma GmbH, Canopy Grouth, Columbia Care, CTC Communications Corp., Demecan, Eurox Deutschland GmbH, Global Praxis Group Limited, IMC Germany, Lundbeck, Sanity Group, Stadapharm GmbH, Synendos Therapeutics AG, and Tilray. She is an advisory/scientific board member for CannaMedical Pharma GmbH, Bionorica Ethics GmbH, CannaXan GmbH, Canopy Growth, Columbia Care, IMC Germany, Leafly Deutschland GmbH, Sanity Group, Syqe Medical Ltd., Therapix Biosciences Ltd., and Wayland Group. She has received speaker’s fees from Aphria Deutschland GmbH, Almirall, Cogitando GmbH, Emalex, Eurox Deutschland GmbH, Ever pharma GmbH, Meinhardt Congress GmbH, PR Berater, Spectrum Therapeutics GmbH, Takeda GmbH, Tilray, Wayland Group. She has received royalties fromDeutsches Ärzteblatt, Der Neurologie und Psychiater, Elsevier, Medizinisch Wissenschaftliche Verlagsgesellschaft Berlin, and Kohlhammer. She served as a guest editor for Frontiers in Neurology on the research topic “The neurobiology and genetics of Gilles de la Tourette syndrome: new avenues through large-scale collaborative projects”, is an associate editor for “Cannabis and Cannabinoid Research” and an Editorial Board Member of “Medical Cannabis and Cannabinoids” und “MDPI-Reports” and a Scientific board member for “Zeitschrift für Allgemeinmedizin”. NSZ has no conflicts to declare. DC has received financial support from EU (FP7-HEALTH-2011 No. 278367; FP7-PEOPLE-2012-ITN No. 316978), from VCVGz, and from Tourette Association of America. AH has received financial or material research support from the Association Française pour le syndrome de Gilles de la Tourette (AFSGT). He has received consultant's honoraria from Lundbeck and Noema Pharma. VR has received payment for consulting and writing activities from Lilly, Novartis, and Shire Pharmaceuticals, lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals, and Medice Pharma, and support for research from Shire Pharmaceuticals and Novartis. He has carried out clinical trials in cooperation with the Novartis, Shire, Servier and Otsuka companies. PJH has received a honorarium for an advisory board meeting of Shire. NSZ and CV have no conflicts to declare.
Figures
References
-
- Brainstorm C, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, Escott-Price V, Falcone GJ, Gormley P, Malik R, Patsopoulos NA, Ripke S, Wei Z, Yu D, Lee PH, Turley P, Grenier-Boley B, Chouraki V, Kamatani Y, Berr C, Letenneur L, Hannequin D, Amouyel P, Boland A, Deleuze JF, Duron E, Vardarajan BN, Reitz C, Goate AM, Huentelman MJ, Kamboh MI, Larson EB, Rogaeva E, St George-Hyslop P, Hakonarson H, Kukull WA, Farrer LA, Barnes LL, Beach TG, Demirci FY, Head E, Hulette CM, Jicha GA, Kauwe JSK, Kaye JA, Leverenz JB, Levey AI, Lieberman AP, Pankratz VS, Poon WW, Quinn JF, Saykin AJ, Schneider LS, Smith AG, Sonnen JA, Stern RA, Van Deerlin VM, Van Eldik LJ, Harold D, Russo G, Rubinsztein DC, Bayer A, Tsolaki M, Proitsi P, Fox NC, Hampel H, Owen MJ, Mead S, Passmore P, Morgan K, Nothen MM, Rossor M, Lupton MK, Hoffmann P, Kornhuber J, Lawlor B, McQuillin A, Al-Chalabi A, Bis JC, Ruiz A, Boada M, Seshadri S, Beiser A, Rice K, van der Lee SJ, De Jager PL, Geschwind DH, Riemenschneider M, Riedel-Heller S, Rotter JI, Ransmayr G, Hyman BT, Cruchaga C, Alegret M, Winsvold B, Palta P, Farh KH, Cuenca-Leon E, Furlotte N, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018 doi: 10.1126/science.aap8757. - DOI - PMC - PubMed
-
- Cath DC, Hedderly T, Ludolph AG, Stern JS, Murphy T, HartmannCzernecki AV, Robertson MM, Martino D, Munchau A, Rizzo R, Group EG European clinical guidelines for Tourette syndrome and other tic disorders. Part I: assessment. Eur Child Adolesc Psychiatry. 2011;20:155–171. doi: 10.1007/s00787-011-0164-6. - DOI - PMC - PubMed
-
- Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, King RA, Sandor P, McMahon WM, Lyon GJ, Cath DC, Kurlan R, Robertson MM, Osiecki L, Scharf JM, Mathews CA. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiat. 2015;72:325–333. doi: 10.1001/jamapsychiatry.2014.2650. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical