Role of NRF2 in immune modulator expression in developing lung
- PMID: 34245611
- PMCID: PMC8547792
- DOI: 10.1096/fj.202100129RR
Role of NRF2 in immune modulator expression in developing lung
Abstract
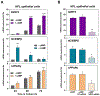
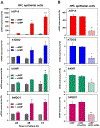
After birth, the alveolar epithelium is exposed to environmental pathogens and high O2 tensions. The alveolar type II cells may protect this epithelium through surfactant production. Surfactant protein, SP-A, an immune modulator, is developmentally upregulated in fetal lung with surfactant phospholipid synthesis. Herein, we observed that the redox-regulated transcription factor, NRF2, and co-regulated C/EBPβ and PPARγ, were markedly induced during cAMP-mediated differentiation of cultured human fetal lung (HFL) epithelial cells. This occurred with enhanced expression of immune modulators, SP-A, TDO2, AhR, and NQO1. Like SP-A, cAMP induction of NRF2 was prevented when cells were exposed to hypoxia. NRF2 knockdown inhibited induction of C/EBPβ, PPARγ, and immune modulators. Binding of endogenous NRF2 to promoters of SP-A and other immune modulator genes increased during HFL cell differentiation. In mouse fetal lung (MFL), a developmental increase in Nrf2, SP-A, Tdo2, Ahr, and Nqo1 and decrease in Keap1 occurred from 14.5 to 18.5 dpc. Developmental induction of Nrf2 in MFL was associated with increased nuclear localization of NF-κB p65, a decline in p38 MAPK phosphorylation, increase in the MAPK phosphatase, DUSP1, induction of the histone acetylase, CBP, and decline in the histone deacetylase, HDAC4. Thus, together with surfactant production, type II cells protect the alveolar epithelium through increased expression of NRF2 and immune modulators to prevent inflammation and oxidative stress. Our findings further suggest that lung cancer cells have usurped this developmental pathway to promote immune tolerance and enhance survival.
Keywords: NRF2; SP-A; development; fetal lung; immune modulators; oxygen tension.
© 2021 Federation of American Societies for Experimental Biology.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors state explicitly that there are no conflicts of interest in connection with this article.
Figures





Similar articles
-
NRF2 Serves a Critical Role in Regulation of Immune Checkpoint Proteins (ICPs) During Trophoblast Differentiation.Endocrinology. 2022 Jul 1;163(7):bqac070. doi: 10.1210/endocr/bqac070. Endocrinology. 2022. PMID: 35596653 Free PMC article.
-
Epigenetic regulation of surfactant protein A gene (SP-A) expression in fetal lung reveals a critical role for Suv39h methyltransferases during development and hypoxia.Mol Cell Biol. 2011 May;31(10):1949-58. doi: 10.1128/MCB.01063-10. Epub 2011 Mar 14. Mol Cell Biol. 2011. PMID: 21402781 Free PMC article.
-
The MicroRNA 29 Family Promotes Type II Cell Differentiation in Developing Lung.Mol Cell Biol. 2016 Jul 29;36(16):2141. doi: 10.1128/MCB.00096-16. Print 2016 Aug 15. Mol Cell Biol. 2016. PMID: 27215389 Free PMC article.
-
Hormonal and developmental regulation of pulmonary surfactant synthesis in fetal lung.Baillieres Clin Endocrinol Metab. 1990 Jun;4(2):351-78. doi: 10.1016/s0950-351x(05)80055-2. Baillieres Clin Endocrinol Metab. 1990. PMID: 2248600 Review.
-
Developmental and hormonal regulation of surfactant protein A (SP-A) gene expression in fetal lung.J Dev Physiol. 1991 Jan;15(1):61-9. J Dev Physiol. 1991. PMID: 1651967 Review.
Cited by
-
NRF2 and the Moirai: Life and Death Decisions on Cell Fates.Antioxid Redox Signal. 2023 Mar;38(7-9):684-708. doi: 10.1089/ars.2022.0200. Epub 2023 Feb 22. Antioxid Redox Signal. 2023. PMID: 36509429 Free PMC article. Review.
-
Sex-Specific Alterations of the Lung Transcriptome at Birth in Mouse Offspring Prenatally Exposed to Vanilla-Flavored E-Cigarette Aerosols and Enhanced Susceptibility to Asthma.Int J Environ Res Public Health. 2023 Feb 20;20(4):3710. doi: 10.3390/ijerph20043710. Int J Environ Res Public Health. 2023. PMID: 36834405 Free PMC article.
-
IL-1β mediates the induction of immune checkpoint regulators IDO1 and PD-L1 in lung adenocarcinoma cells.Cell Commun Signal. 2023 Nov 20;21(1):331. doi: 10.1186/s12964-023-01348-1. Cell Commun Signal. 2023. PMID: 37985999 Free PMC article.
-
Dual role of Nrf2 signaling in hepatocellular carcinoma: promoting development, immune evasion, and therapeutic challenges.Front Immunol. 2024 Sep 2;15:1429836. doi: 10.3389/fimmu.2024.1429836. eCollection 2024. Front Immunol. 2024. PMID: 39286246 Free PMC article. Review.
References
-
- Mendelson CR, Chen C, Boggaram V, Zacharias C, Snyder JM. Regulation of the synthesis of the major surfactant apoprotein in fetal rabbit lung tissue. J Biol Chem. July 25 1986;261(21):9938–43. - PubMed
-
- Boggaram V, Qing K, Mendelson CR. The major apoprotein of rabbit pulmonary surfactant. Elucidation of primary sequence and cyclic AMP and developmental regulation. J Biol Chem. February 25 1988;263(6):2939–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

