Plant-phenotypic changes induced by parasitoid ichnoviruses enhance the performance of both unparasitized and parasitized caterpillars
- PMID: 34245612
- PMCID: PMC8518489
- DOI: 10.1111/mec.16072
Plant-phenotypic changes induced by parasitoid ichnoviruses enhance the performance of both unparasitized and parasitized caterpillars
Abstract
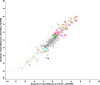
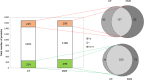
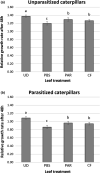
There is increasing awareness that interactions between plants and insects can be mediated by microbial symbionts. Nonetheless, evidence showing that symbionts associated with organisms beyond the second trophic level affect plant-insect interactions are restricted to a few cases belonging to parasitoid-associated bracoviruses. Insect parasitoids harbour a wide array of symbionts which, like bracoviruses, can be injected into their herbivorous hosts to manipulate their physiology and behaviour. Yet, the function of these symbionts in plant-based trophic webs remains largely overlooked. Here, we provide the first evidence of a parasitoid-associated symbiont belonging to the group of ichnoviruses which affects the strength of plant-insect interactions. A comparative proteomic analysis shows that, upon parasitoid injection of calyx fluid containing ichnovirus particles, the composition of salivary glands of caterpillars changes both qualitatively (presence of two viral-encoded proteins) and quantitatively (abundance of several caterpillar-resident enzymes, including elicitors such as glucose oxidase). In turn, plant phenotypic changes triggered by the altered composition of caterpillar oral secretions affect the performance of herbivores. Ichnovirus manipulation of plant responses to herbivory leads to benefits for their parasitoid partners in terms of reduced developmental time within the parasitized caterpillar. Interestingly, plant-mediated ichnovirus-induced effects also enhance the performances of unparasitized herbivores which in natural conditions may feed alongside parasitized ones. We discuss these findings in the context of ecological costs imposed to the plant by the viral symbiont of the parasitoid. Our results provide intriguing novel findings about the role played by carnivore-associated symbionts on plant-insect-parasitoid systems and underline the importance of placing mutualistic associations in an ecological perspective.
Keywords: host-parasitoid interaction; parasitoid-associated symbionts; plant-herbivore-microbe interactions; plant-mediated species interactions; polydnaviruses.
© 2021 The Authors. Molecular Ecology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Indirect plant-mediated interactions between heterospecific parasitoids that develop in different caterpillar species.Oecologia. 2023 Dec;203(3-4):311-321. doi: 10.1007/s00442-023-05465-z. Epub 2023 Oct 27. Oecologia. 2023. PMID: 37889312 Free PMC article.
-
Convergence in Symbiont-Induced Plant-Mediated Responses to Herbivory: Cascading Effects for Foraging Parasitoids.Ecol Lett. 2025 Aug;28(8):e70183. doi: 10.1111/ele.70183. Ecol Lett. 2025. PMID: 40748179 Free PMC article.
-
Parasitic wasp-associated symbiont affects plant-mediated species interactions between herbivores.Ecol Lett. 2018 Jul;21(7):957-967. doi: 10.1111/ele.12952. Epub 2018 Apr 15. Ecol Lett. 2018. PMID: 29656523
-
Influence of parasitoid-associated viral symbionts on plant-insect interactions and biological control.Curr Opin Insect Sci. 2021 Apr;44:64-71. doi: 10.1016/j.cois.2021.03.009. Epub 2021 Apr 15. Curr Opin Insect Sci. 2021. PMID: 33866043 Review.
-
Impact of parasitoid-associated polydnaviruses on plant-mediated herbivore interactions.Curr Opin Insect Sci. 2022 Feb;49:56-62. doi: 10.1016/j.cois.2021.11.004. Epub 2021 Nov 26. Curr Opin Insect Sci. 2022. PMID: 34839032 Review.
Cited by
-
Indirect plant-mediated interactions between heterospecific parasitoids that develop in different caterpillar species.Oecologia. 2023 Dec;203(3-4):311-321. doi: 10.1007/s00442-023-05465-z. Epub 2023 Oct 27. Oecologia. 2023. PMID: 37889312 Free PMC article.
-
Convergence in Symbiont-Induced Plant-Mediated Responses to Herbivory: Cascading Effects for Foraging Parasitoids.Ecol Lett. 2025 Aug;28(8):e70183. doi: 10.1111/ele.70183. Ecol Lett. 2025. PMID: 40748179 Free PMC article.
-
Evolution of koinobiont parasitoid host regulation and consequences for indirect plant defence.Evol Ecol. 2022;36(3):299-319. doi: 10.1007/s10682-022-10180-x. Epub 2022 May 9. Evol Ecol. 2022. PMID: 35663232 Free PMC article. Review.
References
-
- Beckage, N. E. (2012). Polydnaviruses as endocrine regulators. In Beckage N. E. & Drezen J. M. (Eds.), Parasitoid viruses: Symbionts and pathogens (pp. 163–168). Elsevier Academic Press Inc.
-
- Beckage, N. E. , Tan, F. F. , Schleifer, K. W. , Lane, R. D. , & Cherubin, L. L. (1994). Characterization and biological effects of Cotesia congregata polydnavirus on host larvae of the tobacco hornworm, Manduca sexta . Archives of Insect Biochemistry and Physiology, 26(2–3), 165–195. 10.1002/arch.940260209 - DOI
-
- Bitra, K. , Zhang, S. , & Strand, M. R. (2011). Transcriptomic profiling of Microplitis demolitor bracovirus reveals host, tissue and stage‐specific patterns of activity. Journal of General Virology, 92(9), 2060–2071 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources

