A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters
- PMID: 34245672
- PMCID: PMC8238649
- DOI: 10.1016/j.celrep.2021.109400
A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters
Abstract
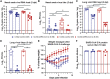
The development of an effective vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiologic agent of coronavirus disease 2019 (COVID-19), is a global priority. Here, we compare the protective capacity of intranasal and intramuscular delivery of a chimpanzee adenovirus-vectored vaccine encoding a prefusion stabilized spike protein (chimpanzee adenovirus [ChAd]-SARS-CoV-2-S) in Golden Syrian hamsters. Although immunization with ChAd-SARS-CoV-2-S induces robust spike-protein-specific antibodies capable of neutralizing the virus, antibody levels in serum are higher in hamsters vaccinated by an intranasal compared to intramuscular route. Accordingly, against challenge with SARS-CoV-2, ChAd-SARS-CoV-2-S-immunized hamsters are protected against less weight loss and have reduced viral infection in nasal swabs and lungs, and reduced pathology and inflammatory gene expression in the lungs, compared to ChAd-control immunized hamsters. Intranasal immunization with ChAd-SARS-CoV-2-S provides superior protection against SARS-CoV-2 infection and inflammation in the upper respiratory tract. These findings support intranasal administration of the ChAd-SARS-CoV-2-S candidate vaccine to prevent SARS-CoV-2 infection, disease, and possibly transmission.
Keywords: ChAd vectored vaccine; SARS-CoV-2; Syrian hamster; intranasal immunization.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.S.D. is a consultant for Inbios, Vir Biotechnology, NGM Biopharmaceuticals, and Carnival Corporation and on the Scientific Advisory Board of Moderna and Immunome. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Moderna, Vir Biotechnology, and Emergent BioSolutions. The Boon laboratory has received unrelated funding support in sponsored research agreements from AI Therapeutics, GreenLight Biosciences Inc., AbbVie Inc., and Nano targeting & Therapy Biopharma Inc. M.S.D. and A.O.H. have filed a disclosure with Washington University for possible commercial development of ChAd-SARS-CoV-2. A.C.M.B. is a recipient of a licensing agreement with Abbvie Inc. for commercial development of SARS-CoV-2 monoclonal antibody (mAb).
Figures




Update of
-
A single intranasal or intramuscular immunization with chimpanzee adenovirus vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters.bioRxiv [Preprint]. 2020 Dec 3:2020.12.02.408823. doi: 10.1101/2020.12.02.408823. bioRxiv. 2020. Update in: Cell Rep. 2021 Jul 20;36(3):109400. doi: 10.1016/j.celrep.2021.109400. PMID: 33299991 Free PMC article. Updated. Preprint.
References
-
- Chan J.F.-W., Zhang A.J., Yuan S., Poon V.K.-M., Chan C.C.-S., Lee A.C.-Y., Chan W.-M., Fan Z., Tsoi H.-W., Wen L. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020;71:2428–2446. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

