Persistence of anti-SARS-CoV-2 antibodies: immunoassay heterogeneity and implications for serosurveillance
- PMID: 34245905
- PMCID: PMC8261139
- DOI: 10.1016/j.cmi.2021.06.040
Persistence of anti-SARS-CoV-2 antibodies: immunoassay heterogeneity and implications for serosurveillance
Abstract
Objectives: Serological studies have been critical in tracking the evolution of the COVID-19 pandemic. Data on anti-SARS-CoV-2 antibodies persistence remain sparse, especially from infected individuals with few to no symptoms. The objective of the study was to quantify the sensitivity for detecting historic SARS-CoV-2 infections as a function of time since infection for three commercially available SARS-CoV-2 immunoassays and to explore the implications of decaying immunoassay sensitivity in estimating seroprevalence.
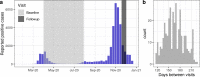
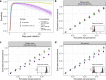
Methods: We followed a cohort of mostly mild/asymptomatic SARS-CoV-2-infected individuals (n = 354) at least 8 months after their presumed infection date and tested their serum for anti-SARS-CoV-2 antibodies with three commercially available assays: Roche-N, Roche-RBD and EuroImmun-S1. We developed a latent class statistical model to infer the specificity and time-varying sensitivity of each assay and show through simulations how inappropriately accounting for test performance can lead to biased serosurvey estimates.
Results: Antibodies were detected at follow-up in 74-100% of participants, depending on immunoassays. Both Roche assays maintain high sensitivity, with the EuroImmun assay missing 40% of infections after 9 months. Simulations reveal that without appropriate adjustment for time-varying assay sensitivity, seroprevalence surveys may underestimate infection rates.
Discussion: Antibodies persist for at least 8 months after infection in a cohort of mildly infected individuals with detection depending on assay choice. Appropriate assay performance adjustment is important for the interpretation of serological studies in the case of diminishing sensitivity after infection.
Keywords: Latent class model; SARS-CoV-2; Seroepidemiology; Seroprevalence; Serosurveillance.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

Similar articles
-
SARS-CoV-2 Antibody Testing in Health Care Workers: A Comparison of the Clinical Performance of Three Commercially Available Antibody Assays.Microbiol Spectr. 2021 Oct 31;9(2):e0039121. doi: 10.1128/Spectrum.00391-21. Epub 2021 Sep 29. Microbiol Spectr. 2021. PMID: 34585976 Free PMC article.
-
Evaluation of Ortho VITROS and Roche Elecsys S and NC Immunoassays for SARS-CoV-2 Serosurveillance Applications.Microbiol Spectr. 2023 Aug 17;11(4):e0323422. doi: 10.1128/spectrum.03234-22. Epub 2023 Jun 22. Microbiol Spectr. 2023. PMID: 37347180 Free PMC article.
-
Evaluation of Two Chemiluminescent and Three ELISA Immunoassays for the Detection of SARS-CoV-2 IgG Antibodies: Implications for Disease Diagnosis and Patients' Management.Front Immunol. 2020 Dec 23;11:609242. doi: 10.3389/fimmu.2020.609242. eCollection 2020. Front Immunol. 2020. PMID: 33424863 Free PMC article.
-
How to interpret and use COVID-19 serology and immunology tests.Clin Microbiol Infect. 2021 Jul;27(7):981-986. doi: 10.1016/j.cmi.2021.05.001. Epub 2021 May 8. Clin Microbiol Infect. 2021. PMID: 33975005 Free PMC article. Review.
-
Strengthening serological studies: the need for greater geographical diversity, biobanking, and data-accessibility.Trends Microbiol. 2025 Jan 15:S0966-842X(24)00322-6. doi: 10.1016/j.tim.2024.12.006. Online ahead of print. Trends Microbiol. 2025. PMID: 39818508 Review.
Cited by
-
Specchio-COVID19 cohort study: a longitudinal follow-up of SARS-CoV-2 serosurvey participants in the canton of Geneva, Switzerland.BMJ Open. 2022 Jan 31;12(1):e055515. doi: 10.1136/bmjopen-2021-055515. BMJ Open. 2022. PMID: 35105645 Free PMC article.
-
Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: a statistical analysis.Lancet. 2022 Jun 25;399(10344):2351-2380. doi: 10.1016/S0140-6736(22)00484-6. Epub 2022 Apr 8. Lancet. 2022. PMID: 35405084 Free PMC article.
-
Seroprevalence of anti-SARS-CoV-2 antibodies in Thai adults during the first three epidemic waves.PLoS One. 2022 Apr 27;17(4):e0263316. doi: 10.1371/journal.pone.0263316. eCollection 2022. PLoS One. 2022. PMID: 35476709 Free PMC article.
-
Seroprevalence of anti-SARS-CoV-2 antibodies 6 months into the vaccination campaign in Geneva, Switzerland, 1 June to 7 July 2021.Euro Surveill. 2021 Oct;26(43):2100830. doi: 10.2807/1560-7917.ES.2021.26.43.2100830. Euro Surveill. 2021. PMID: 34713799 Free PMC article.
-
Systematic literature review of SARS-CoV-2 seroprevalence surveys in Canada through April 2021.IJID Reg. 2022 Sep;4:157-164. doi: 10.1016/j.ijregi.2022.07.010. Epub 2022 Jul 29. IJID Reg. 2022. PMID: 35919829 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

