BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel
- PMID: 34245907
- PMCID: PMC8261136
- DOI: 10.1016/j.cmi.2021.06.036
BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel
Abstract
Objectives: The mRNA coronavirus disease 2019 (COVID-19) vaccines have shown high effectiveness in the prevention of symptomatic COVID-19, hospitalization, severe disease and death. Nevertheless, a minority of vaccinated individuals might become infected and experience significant morbidity. Characteristics of vaccine breakthrough infections have not been studied. We sought to portray the population of Israeli patients, who were hospitalized with COVID-19 despite full vaccination.
Methods: A retrospective multicentre cohort study of 17 hospitals included patients fully vaccinated with Pfizer/BioNTech's BNT162b2 vaccine who developed COVID-19 more than 7 days after the second vaccine dose and required hospitalization. The risk for poor outcome, defined as a composite of mechanical ventilation or death, was assessed.
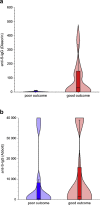
Results: A total of 152 patients were included, accounting for half of hospitalized fully vaccinated patients in Israel. Poor outcome was noted in 38 patients and mortality rate reached 22% (34/152). Notably, the cohort was characterized by a high rate of co-morbidities predisposing to severe COVID-19, including hypertension (108; 71%), diabetes (73; 48%), congestive heart failure (41; 27%), chronic kidney and lung diseases (37; 24% each), dementia (29; 19%) and cancer (36; 24%), and only six (4%) had no co-morbidities. Sixty (40%) of the patients were immunocompromised. Higher viral load was associated with a significant risk for poor outcome. Risk also appeared higher in patients receiving anti-CD20 treatment and in patients with low titres of anti-Spike IgG, but these differences did not reach statistical significance.
Conclusions: We found that severe COVID-19 infection, associated with a high mortality rate, might develop in a minority of fully vaccinated individuals with multiple co-morbidities. Our patients had a higher rate of co-morbidities and immunosuppression compared with previously reported non-vaccinated hospitalized individuals with COVID-19. Further characterization of this vulnerable population may help to develop guidance to augment their protection, either by continued social distancing, or by additional active or passive vaccinations.
Keywords: BNT162b2; Breakthrough infection; Coronavirus disease 2019; Immune compromised; Serology; Severe acute respiratory syndrome coronavirus 2; Vaccine effectiveness; mRNA vaccine.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Ministry of Health COVID-19 dashboard 2021. https://datadashboard.health.gov.il/COVID-19/general Available at:
-
- Haas E., Angulo A., McLaughlin J., Anis E., Singer S., FK K., et al. Nationwide vaccination campaign with BNT162b2 in Israel demonstrates high vaccine effectiveness and marked declines in incidence of SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths. Lancet. 2021 epub ahead of print. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

