Overexpressing miR-122-5p Inhibits the Relaxation of Vaginal Smooth Muscle in Female Sexual Arousal Disorder by Targeting Vasoactive Intestinal Peptide Receptor 1
- PMID: 34246178
- PMCID: PMC8360939
- DOI: 10.1016/j.esxm.2021.100390
Overexpressing miR-122-5p Inhibits the Relaxation of Vaginal Smooth Muscle in Female Sexual Arousal Disorder by Targeting Vasoactive Intestinal Peptide Receptor 1
Erratum in
-
[No title available]Sex Med. 2022 Feb;10(1):100481. doi: 10.1016/j.esxm.2021.100481. Sex Med. 2022. PMID: 35164938 Free PMC article. No abstract available.
Abstract
Introduction: Female sexual arousal disorder (FSAD) is a common issue causing physical and psychological pain, but it has no standard diagnostic criteria or treatment. So its pathogenesis desiderates to be explored.
Aim: To investigate the specific function of miR-122-5p in FSAD.
Methods: 18 subjects were grouped into FSAD and normal control groups according to the Chinese version of the Female Sexual Function Index, and the expression levels of miR-122-5p and vasoactive intestinal peptide receptor 1 (VIPR1) protein in their tissue were verified through real-time quantitative polymerase chain reaction (qRT-PCR) and western blot (WB) analysis. Then in vitro experiment, miR-122-5p was overexpressed or inhibited in rat vaginal smooth muscle cells (SMCs). The relaxation of rat vaginal SMCs was reflected by the cell morphology, intracellular free cytosolic calcium ion (Ca2+) levels, cell proliferation and apoptosis, together with the cyclic adenosine monophosphate (cAMP) concentration and protein kinase A (PKA) activities. Additionally, the expression levels of relaxation-related proteins, including VIPR1, stimulatory G protein (Gs), adenylate cyclase (AC), and PKA, were detected based on WB analysis. Furthermore, a rescue experiment that simultaneously overexpressed or silenced miR-122-5p and VIPR1 was conducted, and all the indicators were evaluated.
Main outcomes measure: The expression level of VIPR1 and downstream proteins, cell morphology, cell proliferation and apoptosis, and intracellular free Ca2+ levels were examined.
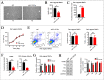
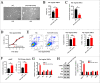
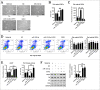
Results: We verified that women with FSAD had higher miR-122-5p and lower VIPR1 protein. Then overexpressing miR-122-5p decreased relaxation of rat vaginal SMCs, which was manifested as a contractile morphology of cells, an increased intracellular free Ca2+ concentration, and lower cAMP concentration and PKA activity. Moreover, by rescue experiments, we inferred that VIPR1 was the target of miR-122-5p and affected the relaxation function of vaginal SMCs.
Conclusion: miR-122-5p regulates the relaxation of vaginal SMCs in FSAD by targeting VIPR1, ulteriorly providing an underlying diagnostic and therapeutic target for FSAD. Cong S, Gui T, Shi Q, et al. Overexpressing miR-122-5p Inhibits the Relaxation of Vaginal Smooth Muscle in Female Sexual Arousal Disorder by Targeting Vasoactive Intestinal Peptide Receptor 1. Sex Med 2021;9:100390.
Keywords: Female sexual arousal disorder; Vaginal smooth muscle cell; Vasoactive intestinal peptide receptor 1; miR-122-5p.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
miR-195-5p Regulates the Phenotype Switch of CCSM Cells by Targeting Smad7.Sex Med. 2021 Jun;9(3):100349. doi: 10.1016/j.esxm.2021.100349. Epub 2021 Jun 1. Sex Med. 2021. PMID: 34087534 Free PMC article.
-
Endogenous vasoactive peptides and the human vagina--a molecular biology and functional study.J Sex Med. 2011 Jan;8(1):35-43. doi: 10.1111/j.1743-6109.2010.01923.x. J Sex Med. 2011. PMID: 20584115
-
MicroRNA MiR-199a-5p regulates smooth muscle cell proliferation and morphology by targeting WNT2 signaling pathway.J Biol Chem. 2015 Mar 13;290(11):7067-86. doi: 10.1074/jbc.M114.618694. Epub 2015 Jan 16. J Biol Chem. 2015. PMID: 25596533 Free PMC article.
-
A review of the physiology and pharmacology of peripheral (vaginal and clitoral) female genital arousal in the animal model.J Urol. 2003 Aug;170(2 Pt 2):S40-4; discussion S44-5. doi: 10.1097/01.ju.0000075352.03144.15. J Urol. 2003. PMID: 12853772 Review.
-
Sildenafil citrate for female sexual arousal disorder: a future possibility?Nat Rev Urol. 2009 Apr;6(4):216-22. doi: 10.1038/nrurol.2009.25. Nat Rev Urol. 2009. PMID: 19352396 Review.
Cited by
-
Knockdown of miR-19a suppresses gastrointestinal dysmotility diarrhea after TBI by regulating VIP expression.Brain Behav. 2023 Jul;13(7):e3071. doi: 10.1002/brb3.3071. Epub 2023 May 22. Brain Behav. 2023. PMID: 37218372 Free PMC article.
References
-
- Nappi RE, Cucinella L, Martella S. Female sexual dysfunction (FSD): prevalence and impact on quality of life (QoL) Maturitas. 2016;94:87–91. - PubMed
-
- Ma J, Pan L, Lei Y. Prevalence of female sexual dysfunction in urban Chinese women based on cutoff scores of the Chinese version of the female sexual function index: a preliminary study. J Sex Med. 2014;11:909–919. - PubMed
-
- Nappi RE, Cucinella L. Advances in pharmacotherapy for treating female sexual dysfunction. Expert Opin Pharmacother. 2015;16:875–887. - PubMed
-
- Zhang C, Tong J, Zhu L. A population-based epidemiologic study of female sexual dysfunction risk in Mainland China: prevalence and predictors. J Sex Med. 2017;14:1348–1356. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

