Efficacy and safety of fremanezumab in patients with episodic and chronic migraine with documented inadequate response to 2 to 4 classes of migraine preventive medications over 6 months of treatment in the phase 3b FOCUS study
- PMID: 34246226
- PMCID: PMC8272284
- DOI: 10.1186/s10194-021-01279-7
Efficacy and safety of fremanezumab in patients with episodic and chronic migraine with documented inadequate response to 2 to 4 classes of migraine preventive medications over 6 months of treatment in the phase 3b FOCUS study
Abstract
Background: Fremanezumab, a fully humanized monoclonal antibody (IgG2Δa) selectively targets the calcitonin gene-related peptide and has proven efficacy for the preventive treatment of migraine. In this study, we evaluated the long-term efficacy, safety, and tolerability of monthly and quarterly fremanezumab.
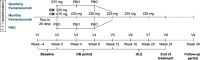
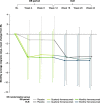
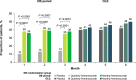
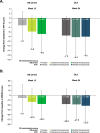
Methods: Episodic migraine and chronic migraine patients completing the 12-week double-blind period of the FOCUS trial entered the 12-week open-label extension and received 3 monthly doses of fremanezumab (225 mg). Changes from baseline in monthly migraine days, monthly headache days of at least moderate severity, days of acute headache medication use, days with photophobia/phonophobia, days with nausea or vomiting, disability scores, and proportion of patients achieving a ≥50% or ≥75% reduction in monthly migraine days were evaluated.
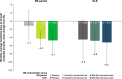
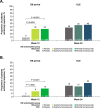
Results: Of the 807 patients who completed the 12-week double-blind treatment period and entered the open-label extension, 772 patients completed the study. In the placebo, quarterly fremanezumab, and monthly fremanezumab dosing regimens, respectively, patients had fewer average monthly migraine days (mean [standard deviation] change from baseline: - 4.7 [5.4]; - 5.1 [4.7]; - 5.5 [5.0]), monthly headache days of at least moderate severity (- 4.5 [5.0]; - 4.8 [4.5]; - 5.2 [4.9]), days per month of acute headache medication use (- 4.3 [5.2]; - 4.9 [4.6]; - 4.8 [4.9]), days with photophobia/phonophobia (- 3.1 [5.3]; - 3.4 [5.3]; - 4.0 [5.2]), and days with nausea or vomiting (- 2.3 [4.6]; - 3.1 [4.5]; - 3.0 [4.4]). During the 12-week open-label extension, 38%, 45%, and 46% of patients, respectively, achieved a ≥50% reduction and 16%, 15%, and 20%, respectively, achieved a ≥75% reduction in monthly migraine days. Disability scores were substantially improved in all 3 treatment groups. There were low rates of adverse events leading to discontinuation (<1%).
Conclusion: Fremanezumab demonstrated sustained efficacy up to 6 months and was well tolerated in patients with episodic migraine or chronic migraine and documented inadequate response to multiple migraine preventive medication classes.
Trial registration: ClinicalTrials.gov NCT03308968 (FOCUS).
Keywords: CGRP; Calcitonin gene-related peptide; Long-term efficacy; Long-term safety; Migraine.
© 2021. The Author(s).
Conflict of interest statement
MA has received personal fees from AbbVie/Allergan, Amgen, Eli Lilly, Lundbeck, Novartis, and Teva Pharmaceuticals. MA is the principal investigator for ongoing AbbVie/Allergan, Amgen and Lundbeck trials. MA has no ownership interest and does not own stocks of any pharmaceutical company. MA serves as associate editor of
Figures


References
-
- Deuschl G, Beghi E, Fazekas F, Varga T, Christoforidi KA, Sipido E, Bassetti CL, Vos T, Feigin VL (2020) The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 5(10):e551–e567. 10.1016/S2468-2667(20)30190-0 - PubMed
-
- Martelletti P, Schwedt TJ, Lanteri-Minet M, Quintana R, Carboni V, Diener HC, Ruiz de la Torre E, Craven A, Rasmussen AV, Evans S, Laflamme AK, Fink R, Walsh D, Dumas P, Vo P (2018) My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 19(1):115. 10.1186/s10194-018-0946-z - PMC - PubMed
-
- American Headache Society The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

