Continuous Blood purification on Influenza-Associated Neurological Disease in children: a retrospective cohort study
- PMID: 34246228
- PMCID: PMC8271303
- DOI: 10.1186/s12879-021-06265-7
Continuous Blood purification on Influenza-Associated Neurological Disease in children: a retrospective cohort study
Abstract
Background: Due to lack of proven therapies, we evaluated the effect of CBP on Influenza-Associated Neurological Disease in children.
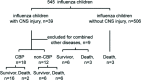
Methods: A single-center, retrospective, cohort study was conducted in Luoyang, Henan province, China from January 2018 to January 2020. Children (<18 years) with influenza-associated neurological disease were enrolled in the study. Children with indications for CBP and parental consent received CBP (Continuous Blood purification), while others received maximal intensive care treatment because of the absence of parental consent. The outcomes of the CBP and non-CBP groups were compared. Categorical variables were presented as percentage and compared by Chi-square test. Continuous variables were expressed as median (interquartile ranges) and compared with non-parametric independent sample test. Statistical analyses were carried out by SPSS (version 26.0) and p < 0.05 (2 tailed) was considered to be statistically significant.
Results: 30 children with influenza-associated neurological disease were recruited to the study. 18 received CBP and the other 12 received maximal intensive care. There were no differences between CBP and non-CBP children in age, sex, body weight, type of influenza virus, neurological complications, Glasgow score, PIM-2 score and PCIS at admission (p > 0.05). The inflammatory factors (CRP, PCT and IL-6) of 30 cases were tested at admission and after 3 days of admission. In the CBP group, there was a significant decrease in IL-6 levels at 3 days of admission (p = 0.003) and a decrease in CRP and PCT levels, but no significant difference (p > 0.05). In the non-CBP group, there were no significant difference on levels of CRP, PCT and IL-6 at admission and 3-day of admission (p > 0.05). The 28-day mortality was significantly lower in the CBP group compared with the non-CBP group (11.11% vs. 50%, p = 0.034).
Conclusions: CBP definitely reduces IL-6 levels significantly. We did find that the survival rate of patients in the CBP group was improved. But we don't know if there is a relationship between the reduction of IL-6 levels and the survival rate.
Trial registration: http://www.chictr.org.cn/index.aspx (ChiCTR2000031754).
Keywords: Children; Continuous blood purification; Influenza; Neurological complications; Retrospective cohort study.
© 2021. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest or funding to disclose.
Figures

References
-
- World Health Organization. Influenza (seasonal). [Cited 19 April 2018]. 2018. http://www.who.int/mediacentre/factsheets/fs211/en/.
-
- Ishida Y, Kawashima H, Morichi S, et al. Brain magnetic resonance imaging in acute phase of pandemic influenza A (H1N1) 2009–associated encephalopathy in children. Neuropediatrics. 2015;46(1):20–5. - PubMed
MeSH terms
Grants and funding
- LHGJ20191235/The efficacy and mechanism of continuous blood purification in the treatment of children's Influenza-associated neurological disease
- QN-2019-B15/The plasma exchange combined with hemofiltration on the clearing effect mechanism of YKL-40, IL-6, IL-10 and TNF-a in children with severe viral encephalitis
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

