isoSTED microscopy with water-immersion lenses and background reduction
- PMID: 34246627
- PMCID: PMC8392127
- DOI: 10.1016/j.bpj.2021.05.031
isoSTED microscopy with water-immersion lenses and background reduction
Abstract
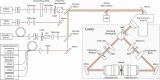
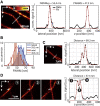
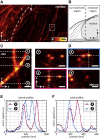
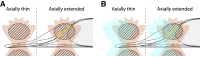
Fluorescence microscopy is an excellent tool to gain knowledge on cellular structures and biochemical processes. Stimulated emission depletion (STED) microscopy provides a resolution in the range of a few 10 nm at relatively fast data acquisition. As cellular structures can be oriented in any direction, it is of great benefit if the microscope exhibits an isotropic resolution. Here, we present an isoSTED microscope that utilizes water-immersion objective lenses and enables imaging of cellular structures with an isotropic resolution of better than 60 nm in living samples at room temperature and without CO2 supply or another pH control. This corresponds to a reduction of the focal volume by far more than two orders of magnitude as compared to confocal microscopy. The imaging speed is in the range of 0.8 s/μm3. Because fluorescence signal can only be detected from a diffraction-limited volume, a background signal is inevitably observed at resolutions well beyond the diffraction limit. Therefore, we additionally present a method that allows us to identify this unspecific background signal and to remove it from the image.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


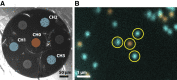
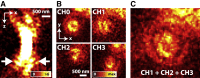


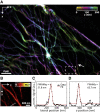
Comment in
-
Extending the performance capabilities of isoSTED.Biophys J. 2021 Aug 17;120(16):3237-3239. doi: 10.1016/j.bpj.2021.07.005. Epub 2021 Jul 9. Biophys J. 2021. PMID: 34348101 Free PMC article. No abstract available.
References
-
- Hell S.W. Microscopy and its focal switch. Nat. Methods. 2009;6:24–32. - PubMed
-
- Hell S.W., Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994;19:780–782. - PubMed
-
- Hell S.W., Kroug M. Ground-state-depletion fluorescence microscopy: a concept for breaking the diffraction resolution limit. Appl. Phys. B. 1995;60:495–497.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

