The ketone body β-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults
- PMID: 34246657
- PMCID: PMC8889914
- DOI: 10.1016/j.kint.2021.06.031
The ketone body β-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults
Erratum in
-
Corrigendum to Fang Y, Chen B, Gong AY, et al. "The ketone body β-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults." Kidney Int. 2021;100:1037-1053.Kidney Int. 2022 Jun;101(6):1301-1302. doi: 10.1016/j.kint.2022.04.002. Kidney Int. 2022. PMID: 35597599 Free PMC article. No abstract available.
Abstract
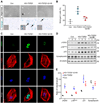
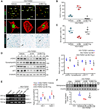
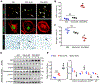
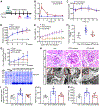
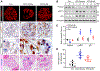
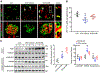
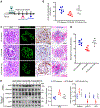
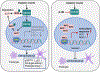
Diabetic kidney disease (DKD) is one of the most common complications of diabetes and is clinically featured by progressive albuminuria, consequent to glomerular destruction that involves podocyte senescence. Burgeoning evidence suggests that ketosis, in particular β-hydroxybutyrate, exerts a beneficial effect on aging and on myriad metabolic or chronic diseases, including obesity, diabetes and chronic kidney diseases. Its effect on DKD is largely unknown. In vitro in podocytes exposed to a diabetic milieu, β-hydroxybutyrate treatment substantially mitigated cellular senescence and injury, as evidenced by reduced formation of γH2AX foci, reduced staining for senescence-associated-β-galactosidase activity, diminished expression of key mediators of senescence signaling like p16INK4A and p21, and preserved expression of synaptopodin. This beneficial action of β-hydroxybutyrate coincided with a reinforced transcription factor Nrf2 antioxidant response. Mechanistically, β-hydroxybutyrate inhibition of glycogen synthase kinase 3β (GSK3β), a convergent point for myriad signaling pathways regulating Nrf2 activity, seems to contribute. Indeed, trigonelline, a selective inhibitor of Nrf2, or ectopic expression of constitutively active mutant GSK3β abolished, whereas selective activation of Nrf2 was sufficient for the anti-senescent and podocyte protective effects of β-hydroxybutyrate. Moreover, molecular modeling and docking analysis revealed that β-hydroxybutyrate is able to directly target the ATP-binding pocket of GSK3β and thereby block its kinase activity. In murine models of streptozotocin-elicited DKD, β-hydroxybutyrate therapy inhibited GSK3β and reinforced Nrf2 activation in glomerular podocytes, resulting in lessened podocyte senescence and injury and improved diabetic glomerulopathy and albuminuria. Thus, our findings may pave the way for developing a β-hydroxybutyrate-based novel approach of therapeutic ketosis for treating DKD.
Keywords: GSK3β; Nrf2 antioxidant response; aging; diabetic nephropathy; intermittent fasting; ketosis; time-restricted feeding.
Copyright © 2021 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
All the authors declared no competing interests.
Figures



References
-
- Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014; 370: 1514–1523. - PubMed
-
- Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018. Am J Kidney Dis 2018; 71: 884–895. - PubMed
-
- de Cabo R, Mattson MP. Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med 2019; 381: 2541–2551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

