Systematic Review and Subgroup Meta-analysis of Randomized Trials to Determine Tocilizumab's Place in COVID-19 Pneumonia
- PMID: 34247325
- PMCID: PMC8272840
- DOI: 10.1007/s40121-021-00488-6
Systematic Review and Subgroup Meta-analysis of Randomized Trials to Determine Tocilizumab's Place in COVID-19 Pneumonia
Abstract
Introduction: Tocilizumab randomized clinical trial results are heterogeneous because of the heterogenous population included in them.
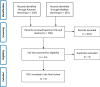
Methods: We conducted a meta-analysis with subgroup meta-analysis (PRISMA guidelines) between severe and non-severe COVID-19.
Results: We included nine trials. Overall, the mortality rate was 24.5% (821/3357) in the tocilizumab group and 29.1% (908/3125) in the control group at day 28-30 (pooled OR, 0.85; 95% CI 0.76-0.96; p = 0.006). Considering the subgroup analysis, this benefit on mortality was confirmed and amplified in the severe COVID-19 group (pooled OR, 0.82; 95% CI 0.73-0.93; p = 0.001) but not in the non-severe COVID-19 group (pooled OR, 1.46; 95% CI 0.91-2.34; p = 0.12). For patients who were not mechanically ventilated at baseline (5523/6482), the pooled OR (0.74; 95% CI 0.64-0.85; p < 0.0001) for mechanical ventilation incidence at day 28-30 was in favor of tocilizumab (cumulative incidence of 14.8% versus 19.4% in tocilizumab and control arm, respectively). This benefit was confirmed in both subgroups, i.e., severe and non-severe COVID-19.
Conclusion: Tocilizumab is an effective treatment in hospitalized patients with COVID-19 and hypoxemia by improving survival and decreasing mechanical ventilation requirement. The greatest benefit is observed in severe COVID-19.
Keywords: Coronavirus disease 2019; Meta-analysis; Randomized clinical trial; Review; Tocilizumab.
© 2021. The Author(s).
Figures







References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int. Accessed 5 May 2021.
-
- RECOVERY Collaborative Group, Horby PW, Pessoa-Amorim G, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. 10.1101/2021.02.11.21249258v1. Accessed 11 Mar 2021.
Publication types
LinkOut - more resources
Full Text Sources

