FoxO3 deficiency in cortical astrocytes leads to impaired lipid metabolism and aggravated amyloid pathology
- PMID: 34247441
- PMCID: PMC8373366
- DOI: 10.1111/acel.13432
FoxO3 deficiency in cortical astrocytes leads to impaired lipid metabolism and aggravated amyloid pathology
Abstract
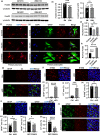
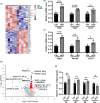
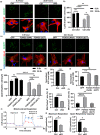
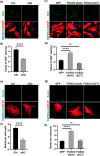
The rise of life expectancy of the human population is accompanied by the drastic increases of age-associated diseases, in particular Alzheimer's disease (AD), and underscores the need to understand how aging influences AD development. The Forkhead box O transcription factor 3 (FoxO3) is known to mediate aging and longevity downstream of insulin/insulin-like growth factor signaling across species. However, its function in the adult brain under physiological and pathological conditions is less understood. Here, we report a region and cell-type-specific regulation of FoxO3 in the central nervous system (CNS). We found that FoxO3 protein levels were reduced in the cortex, but not hippocampus, of aged mice. FoxO3 was responsive to insulin/AKT signaling in astrocytes, but not neurons. Using CNS Foxo3-deficient mice, we reveal that loss of FoxO3 led to cortical astrogliosis and altered lipid metabolism. This is associated with impaired metabolic homoeostasis and β-amyloid (Aβ) uptake in primary astrocyte cultures. These phenotypes can be reversed by expressing a constitutively active FOXO3 but not a FOXO3 mutant lacking the transactivation domain. Loss of FoxO3 in 5xFAD mice led to exacerbated Aβ pathology and synapse loss and altered local response of astrocytes and microglia in the vicinity of Aβ plaques. Astrocyte-specific overexpression of FOXO3 displayed opposite effects, suggesting that FoxO3 functions cell autonomously to mediate astrocyte activity and also interacts with microglia to address Aβ pathology. Our studies support a protective role of astroglial FoxO3 against brain aging and AD.
Keywords: Alzheimer's disease; FoxO3; aging; astrocytes; mice; β-amyloid.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures
Similar articles
-
ApoA-I deficiency increases cortical amyloid deposition, cerebral amyloid angiopathy, cortical and hippocampal astrogliosis, and amyloid-associated astrocyte reactivity in APP/PS1 mice.Alzheimers Res Ther. 2019 May 13;11(1):44. doi: 10.1186/s13195-019-0497-9. Alzheimers Res Ther. 2019. PMID: 31084613 Free PMC article.
-
Blockade of the Interaction of Calcineurin with FOXO in Astrocytes Protects Against Amyloid-β-Induced Neuronal Death.J Alzheimers Dis. 2016 Apr 12;52(4):1471-8. doi: 10.3233/JAD-160149. J Alzheimers Dis. 2016. PMID: 27079728
-
Hippocampal disruptions of synaptic and astrocyte metabolism are primary events of early amyloid pathology in the 5xFAD mouse model of Alzheimer's disease.Cell Death Dis. 2021 Oct 16;12(11):954. doi: 10.1038/s41419-021-04237-y. Cell Death Dis. 2021. PMID: 34657143 Free PMC article.
-
Pathological phenotypes of astrocytes in Alzheimer's disease.Exp Mol Med. 2024 Feb;56(1):95-99. doi: 10.1038/s12276-023-01148-0. Epub 2024 Jan 4. Exp Mol Med. 2024. PMID: 38172603 Free PMC article. Review.
-
Astrocyte and Alzheimer's disease.J Neurol. 2017 Oct;264(10):2068-2074. doi: 10.1007/s00415-017-8593-x. Epub 2017 Aug 18. J Neurol. 2017. PMID: 28821953 Review.
Cited by
-
The role of glial autophagy in Alzheimer's disease.Mol Psychiatry. 2023 Nov;28(11):4528-4539. doi: 10.1038/s41380-023-02242-5. Epub 2023 Sep 7. Mol Psychiatry. 2023. PMID: 37679471 Review.
-
Interrogating mediators of single-cell transcriptional changes in the acute damaged cerebral cortex: Insights into endothelial-astrocyte interactions.Mol Cell Neurosci. 2025 Jun;133:104003. doi: 10.1016/j.mcn.2025.104003. Epub 2025 Mar 14. Mol Cell Neurosci. 2025. PMID: 40090391
-
Astrocytes Lingering at a Crossroads: Neuroprotection and Neurodegeneration in Neurocognitive Dysfunction.Int J Biol Sci. 2025 Apr 28;21(7):3122-3143. doi: 10.7150/ijbs.109315. eCollection 2025. Int J Biol Sci. 2025. PMID: 40384856 Free PMC article. Review.
-
The Role of Astrocytes in Synapse Loss in Alzheimer's Disease: A Systematic Review.Front Cell Neurosci. 2022 Jun 16;16:899251. doi: 10.3389/fncel.2022.899251. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35783099 Free PMC article.
-
Role of FoxO transcription factors in aging and age-related metabolic and neurodegenerative diseases.Cell Biosci. 2021 Nov 2;11(1):188. doi: 10.1186/s13578-021-00700-7. Cell Biosci. 2021. PMID: 34727995 Free PMC article. Review.
References
-
- Alarcón, R., Fuenzalida, C., Santibáñez, M., & von Bernhardi, R. (2005). Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface‐bound beta‐amyloid. Journal of Biological Chemistry, 280(34), 30406–30415. 10.1074/jbc.M414686200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

