International Classification of Retinopathy of Prematurity, Third Edition
- PMID: 34247850
- PMCID: PMC10979521
- DOI: 10.1016/j.ophtha.2021.05.031
International Classification of Retinopathy of Prematurity, Third Edition
Abstract
Purpose: The International Classification of Retinopathy of Prematurity is a consensus statement that creates a standard nomenclature for classification of retinopathy of prematurity (ROP). It was initially published in 1984, expanded in 1987, and revisited in 2005. This article presents a third revision, the International Classification of Retinopathy of Prematurity, Third Edition (ICROP3), which is now required because of challenges such as: (1) concerns about subjectivity in critical elements of disease classification; (2) innovations in ophthalmic imaging; (3) novel pharmacologic therapies (e.g., anti-vascular endothelial growth factor agents) with unique regression and reactivation features after treatment compared with ablative therapies; and (4) recognition that patterns of ROP in some regions of the world do not fit neatly into the current classification system.
Design: Review of evidence-based literature, along with expert consensus opinion.
Participants: International ROP expert committee assembled in March 2019 representing 17 countries and comprising 14 pediatric ophthalmologists and 20 retinal specialists, as well as 12 women and 22 men.
Methods: The committee was initially divided into 3 subcommittees-acute phase, regression or reactivation, and imaging-each of which used iterative videoconferences and an online message board to identify key challenges and approaches. Subsequently, the entire committee used iterative videoconferences, 2 in-person multiday meetings, and an online message board to develop consensus on classification.
Main outcome measures: Consensus statement.
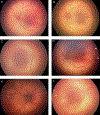
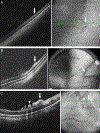
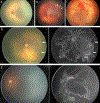
Results: The ICROP3 retains current definitions such as zone (location of disease), stage (appearance of disease at the avascular-vascular junction), and circumferential extent of disease. Major updates in the ICROP3 include refined classification metrics (e.g., posterior zone II, notch, subcategorization of stage 5, and recognition that a continuous spectrum of vascular abnormality exists from normal to plus disease). Updates also include the definition of aggressive ROP to replace aggressive-posterior ROP because of increasing recognition that aggressive disease may occur in larger preterm infants and beyond the posterior retina, particularly in regions of the world with limited resources. ROP regression and reactivation are described in detail, with additional description of long-term sequelae.
Conclusions: These principles may improve the quality and standardization of ROP care worldwide and may provide a foundation to improve research and clinical care.
Keywords: Neonatology; Pediatric ophthalmology; Prematurity; Retina; Retinopathy of prematurity.
Published by Elsevier Inc.
Conflict of interest statement
J. Peter Campbell receives research support from Genentech (South San Francisco, CA).
Antonio Capone Jr is an equity owner of Phoenix Technology Group, LLC, a founder and equity owner of Retinal Solutions, LLC and receives research support from AURA Biosciences (Cambridge, MA), Bayer (Leverkusen, Germany), Genentech (South San Francisco, CA), Ionis Pharmaceuticals (Carlsbad, CA), Novartis (Basel, Switzerland) and Regeneron Pharmaceuticals (Tarrytown, NY).
R. V. Paul Chan is on the Scientific Advisory Board for Phoenix Technology (Fremont, CA), a Consultant for Alcon (Ft. Worth, TX), and a Consultant for Novartis (Basel, Switzerland).
Michael F. Chiang was previously a Consultant for Novartis (Basel, Switzerland), an equity owner of InTeleretina (Honolulu, HI), and received research support from Genentech (South San Francisco, CA).
Alistair Fielder is a consultant for Novartis (Basel, Switzerland) and Bayer (Reading, United Kingdom)
Brian Fleck is a consultant for Novartis (Basel, Switzerland).
Mary Elizabeth Hartnett receives research support from the National Institutes of Health (R01EY017011, F01EY01730, EY014800), and is a consultant for Regeneron (Tarrytown, NY).
Domenico Lepore is a Consultant for Novartis (Basel, Switzerland) and Bayer (Leverkusen, Germany).
Şengül Özdek is a consultant for Novartis (Basel, Switzerland), Bayer (Leverkusen, Germany), and Allergan (Dublin, Ireland).
Andreas Stahl receives research support from Novartis (Basel, Switzerland), and is a Consultant for Novartis (Basel, Switzerland) and Bayer (Leverkusen, Germany).
Cynthia A. Toth receives research support from the National Institutes of Health (R01EY025009, U01EY028079, P30EY005722) and from a Research to Prevent Blindness Stein Award, royalties from Alcon (Fort Worth, TX), and is a founding and equity owner of Theia Imaging, LLC.
Wei-Chi Wu is a consultant for Novartis (Basel, Switzerland), Bayer (Leverkusen, Germany), and Allergan (Dublin, Ireland).
Dr. Campbell is supported by research funding from the National Institutes of Health (R01EY19474, K12EY27720), the National Science Foundation (SCH-1622679), and Genentech (South San Francisco, CA). Dr. Capone is an equity owner of Phoenix Technology Group, LLC, a founder and equity owner of Retinal Solutions, LLC and receives research support from AURA Biosciences (Cambridge, MA), Bayer (Leverkusen, Germany), Genentech (South San Francisco, CA), Ionis Pharmaceuticals (Carlsbad, CA), Novartis (Basel, Switzerland) and Regeneron Pharmaceuticals (Tarrytown, NY). Dr. Fleck is a consultant for Novartis (Basel, Switzerland). Dr. Hartnett receives research support from the National Institutes of Health (R01EY017011, F01EY01730, EY014800), and is a consultant for Regeneron (Tarrytown, NY). Dr. Lepore is a Consultant for Novartis (Basel, Switzerland) and Bayer (Leverkusen, Germany). Dr. Özdek is a consultant for Novartis (Basel, Switzerland), Bayer (Leverkusen, Germany), and Allergan (Dublin, Ireland). Dr. Stahl receives research support from Novartis (Basel, Switzerland), and is a Consultant for Novartis (Basel, Switzerland) and Bayer (Leverkusen, Germany). Dr. Toth receives research support from the National Institutes of Health (R01EY025009, U01EY028079, P30EY005722) and from a Research to Prevent Blindness Stein Award, royalties from Alcon (Fort Worth, TX), and is a founding and equity owner of Theia Imaging, LLC. Dr. Wu is a consultant for Novartis (Basel, Switzerland), Bayer (Leverkusen, Germany), and Allergan (Dublin, Ireland).
Figures
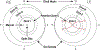







Comment in
-
A Revision of the International Classification of Retinopathy of Prematurity.Ophthalmology. 2021 Oct;128(10):1381-1383. doi: 10.1016/j.ophtha.2021.07.014. Epub 2021 Jul 29. Ophthalmology. 2021. PMID: 34332760 No abstract available.
-
Reply.Ophthalmology. 2022 Mar;129(3):e36-e37. doi: 10.1016/j.ophtha.2021.10.020. Epub 2021 Nov 26. Ophthalmology. 2022. PMID: 34844763 No abstract available.
-
Re: Chiang et al.: International Classification of Retinopathy of Prematurity, Third Edition (Ophthalmology. 2021;128:e51-e68).Ophthalmology. 2022 Mar;129(3):e36. doi: 10.1016/j.ophtha.2021.10.021. Epub 2021 Nov 26. Ophthalmology. 2022. PMID: 34844764 No abstract available.
-
Re: Chiang et al.: International Classification of Retinopathy of Prematurity: Third Edition (Ophthalmology. 2021;128:e51-e68).Ophthalmology. 2022 Jun;129(6):e64-e65. doi: 10.1016/j.ophtha.2022.01.025. Epub 2022 Mar 4. Ophthalmology. 2022. PMID: 35256217 No abstract available.
References
-
- Reese AB, King MJ, Owens WC. Classification of retrolental fibroplasia. Am J Ophthalmol. 1953;36(10):1333–1335. - PubMed
-
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102(8):1130–1134. - PubMed
-
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1988;106(4):471–479. - PubMed
-
- ICROP Committee for classification of late stages of ROP: An international classification of retinopathy of prematurity: II The classification of retinal detachment. Arch Ophthalmol. 1987;105:906–912. - PubMed
-
- Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity Revisited. Arch Ophthal. 2005;123(7):991. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

