AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2021
- PMID: 34248463
- PMCID: PMC8248779
- DOI: 10.1159/000516420
AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2021
Conflict of interest statement
M. Thill: Advisory boards: Amgen, AstraZeneca, Celgene, ClearCut, Clovis, Daiichi Sankyo, Exact Sciences, GSK, Lilly, MSD, Neodynamics, Novartis, Pfizer, pfm medical, Pierre Fabre, Roche, Sysmex, Tesaro. Manuscript support: Amgen, Celgene, Clearcut, Roche. Travel reimbursement: Amgen, art tempi, AstraZeneca, Celgene, Clovis, Connect Medica, Daiichi Sankyo, Eisai, Exact Sciences, Hexal, I-Med Institute, Lilly, MCI, Medtronic, MSD, Novartis, Pfizer, pfm medical, Roche, Sysmex, Tesaro. Congress support: Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Hexal, Novartis, Pfizer, Roche. Lectures: Amgen, art tempi, AstraZeneca, Celgene, Clovis, Connect Medica, Daiichi Sankyo, Exact Sciences, Hexal, I-Med Institute, Lilly, MCI, Medtronic, MSD, Novartis, Pfizer, pfm medical, Roche, Seagen, Sysmex, Tesaro, Vifor. Trial funding: Endomagnetics, Exact Sciences. M. Friedrich: Roche, Pfizer, AstraZeneca, Novartis. C. Kolberg-Liedtke: Advisory boards: Phaon Scientific, Novartis, Pfizer, SurgVision, Carl Zeiss Meditec, Amgen, Onkowissen. Lectures: Roche, Novartis, Pfizer, Lilly, Genomic Health, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, TEVA, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Theramex. Travel grants: Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo. U.-S. Albert: Lectures and/or consulting: Medexo GmbH, Institut für Versicherungsmedizin, Pfizer. M. Banys-Paluchowski: Pfizer, Roche, Novartis, Eli Lilly. I. Bauerfeind: Aurikamed, J. Eickeler. J.-U. Blohmer: Honoraria: Amgen, AstraZeneca, Genomic Health Recipient, MSD Oncology, Myriad Genetics, Novartis, Pfizer, Roche, Sonoscape. Travel, accommodations, expenses: Pfizer, Roche. W. Budach: No conflicts of interest. P. Dall: Advisory boards: Olympus, Roche, Novartis, Tesaro. Congress support: Roche. Lectures: Amgen, Roche. E.M. Fallenberg: GE Healthcare, Siemens, ECR, EUSOBI, ESOR, KCR, Esor, DFG. P.A. Fasching: Grants: Novartis, BioNTech, Cepheid. Personal fees: Novartis, Roche, Pfizer, Celgene, Daiichi Sankyo, AstraZeneca, MSD, Myelo Therapeutics, Macrogenics, Eisai, Puma, Lilly. T. Fehm: AstraZeneca, Celgene, Pfizer, Novartis, Roche, Teva, Daiichi Sankyo. B. Gerber: No conflicts of interest. O. Gluz: Advisory boards/lectures: Celgene, Roche, Genomic Health, Amgen, Pfizer, Novartis, Lilly, Nanostring, Eisai, MSD Travel. Grants: Celgene, Roche, Daiichi Sankyo. N. Harbeck: Honoraria for lectures and/or consulting: Agendia, Amgen, Astra Zeneca, BMS, Celgene, Daiichi Sankyo, Genomic Health, Lilly, MSD, Novartis, Odonate, Pierre Fabre, Pfizer, Roche, Sandoz/Hexal, Seattle Genetics. Minority share holder: Westdeutsche Studiengruppe. J. Heil: No conflicts of interest. J. Huober: Trial funding: Celgene, Novartis, Hexal. Lectures: Lilly, Novartis, Roche, Pfizer, AstraZeneca, MSD, Celgene, Eisai, Abbvie. Advisory boards: Lilly, Novartis, Roche, Pfizer, Hexal, AstraZeneca, MSD, Celgene, Abbvie. Travel expenses: Roche, Pfizer, Novartis, Celgene, Daiichi Sankyo. C. Jackisch: Roche, AstraZeneca, Novartis. H.-H. Kreipe: Reimbursement for attending symposia: Ventana. Other expenses (advisory boards, lectures): Amgen, AstraZeneca, Genomic Health, Lilly, Roche. D. Krug: MSD. T. Kühn: Celgene, Roche, Pfizer. S. Kümmel: Roche, Genentech, Novartis, AstraZeneca, Amgen, Celgene, SOMATEX, Daiichi Sankyo, Puma Biotechnology, pfm medical, Pfizer, MSD Oncology. S. Loibl: Abbvie, Amgen, AstraZeneca, Celgene, Novartis, Pfizer, Roche, Seattle Genetics, PriME/Medscape, Chugai, Teva, Vifor, Daiichi Sankyo, Lilly, Samsung, Eirgenix, BMS, Puma, MSD, Immunomedics. D. Lüftner: Advisory boards/lectures: Amgen, AstraZeneca, Celgene, GSK, Lilly, L'Oréal, MSD, Mundipharma, Mylan, Novartis, Pfizer, Roche, Teva, Tesaro. M.P. Lux: Advisory boards, lectures, travel support: Lilly, Pfizer, Roche, MSD, Hexal, Novartis, AstraZeneca, Eisai, medac, Genomic Health. N. Maass: No conflicts of interest. C. Mundhenke: Not specified. U. Nitz: Not specified. T.-W. Park-Simon: Advisory role or expert testimony and lecture honoraria: Roche, AstraZeneca, Tesaro, Pfizer, Daiichi Sankyo, Lilly, MSD. Participation in clinical trials: Roche, AstraZeneca, Tesaro, Pfizer, Daiichi Sankyo, Lilly, MSD, Novartis, Seattle Genetics. Other financial relationships such as travel: Roche, AstraZeneca, Tesaro, Pfizer, Lilly, MSD, Novartis. T. Reimer: Grants: German Cancer Aid, Else Kröner-Fresenius-Stiftung. Personal fees: Pfizer, Roche, Novartis, AstraZeneca, outside the submitted work. K. Rhiem: AstraZeneca, Tesaro, Pfizer, Roche. A. Rody: Roche, Pfizer, Novartis, Celgen, Genomic Health/Exact Sciences, AstraZeneca, Eisai, MSD, Hexal, Amgen. M. Schmidt: Personal fees: AstraZeneca, Roche, Lilly, SeaGen, MSD, Eisai. Grants: Pierre Fabre, Pantarhei Bioscience, BioNTech, Genentech outside the submitted work. Patent issued for EP 2390370 B1 and EP 2951317 B1. A. Schneeweiss: Grants: Celgene, Roche, Abbvie, Molecular Partner. Personal fees: Roche, AstraZeneca, Celgene, Pfizer, Novartis, MSD, Tesaro, Lilly. F. Schütz: Amgen, AstraZeneca, Lilly, Hexal, MSD, Novartis, Pfizer, Roche, Onkozert, Eickeler Kongressorganisation. H.-P. Sinn: No conflicts of interest related to the submitted manuscript. C. Solbach: Travel, accommodation: Roche, Novartis, AstraZeneca, Amgen, Celgene, Hexal, Daiichi Sankyo, Dialog Service GmbH, Lilly, Pfizer, MSD Oncology, ESAI, Gedon Richter, Mylan, Tesaro. E.-F. Solomayer: Roche, AstraZeneca, Pfizer, Amgen, Celgen, Tesaro, Storz, Erbe, Gedeon Richter, Eisai, Medac, MSD, Vifor, Teva, Ethikon. E. Stickeler: Advisory boards: Novartis, Pfizer, AstraZeneca, Roche, Tesoro. Fees: Novartis, Pfizer, AstraZeneca, Roche. C. Thomssen: Advisory boards, lectures: Amgen, AstraZeneca, Celgen, Daiichi Sankyo, Eisai, Lilly, MSD, Mundipharma, Medapharm, Novartis, Pfizer, Pierre Fabre, Roche, Tesaro, Vifor. M. Untch: presentations, travel grants, all paid to his institution by Abbvie, Amgen, AstraZeneca, BMS, Celgene, Daiichi Sankyo, Eisai, Janssen Cilag, Johnson & Johnson, Lilly Deutschland, Lilly Int., MSD, Mundipharma, Myriad Genetics GmbH Zürich, Odonate, Pfizer GmbH Berlin, Puma Biotechnology, Riemser, Roche, Sanofi Aventis Deutschland GmbH, Sividon Diagnostics Köln, TEVA Pharmaceuticals Ind. Ltd. Berlin. I. Witzel: Not specified. A. Wöckel: Amgen, AstraZeneca, Aurikamed, Celgene, Eisai, Lilly, Novartis, Pfizer, Roche, Tesaro. V. Müller: Speaker honoraria: Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Pfizer, MSD, Novartis, Roche, Teva, Seattle Genetics Consultancy honoraria: Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Tesaro, Nektar. Institutional research support: Novartis, Roche, Seattle Genetics, Genentech. W. Janni: Research grants and/or honoraria: Sanofi-Aventis, Novartis, Roche, Pfizer, AstraZeneca, Chugai, GSK, Eisai, Celgene, Lilly, Janssen, Menarini. N. Ditsch: MSD, Novartis, Pfizer, Roche, AstraZeneca, TEVA, Mentor, MCI Healthcare.
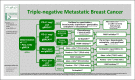
Figures
References
-
- Empfehlungen Gynäkologische Onkologie Kommission Mamma 2021. www.ago-online.de.
-
- Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30((4)):558–66. - PMC - PubMed
-
- Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, et al. TBCRC 048: Phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020 Dec;38((36)):4274–82. - PubMed
-
- André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380((20)):1929–40. - PubMed
Publication types
LinkOut - more resources
Full Text Sources