The Enigmatic Reissner's Fiber and the Origin of Chordates
- PMID: 34248511
- PMCID: PMC8261243
- DOI: 10.3389/fnana.2021.703835
The Enigmatic Reissner's Fiber and the Origin of Chordates
Abstract
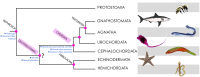
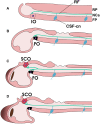
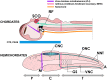
Reissner's fiber (RF) is a secreted filament that floats in the neural canal of chordates. Since its discovery in 1860, there has been no agreement on its primary function, and its strong conservation across chordate species has remained a mystery for comparative neuroanatomists. Several findings, including the chemical composition and the phylogenetic history of RF, clinical observations associating RF with the development of the neural canal, and more recent studies suggesting that RF is needed to develop a straight vertebral column, may shed light on the functions of this structure across chordates. In this article, we will briefly review the evidence mentioned above to suggest a role of RF in the origin of fundamental innovations of the chordate body plan, especially the elongation of the neural tube and maintenance of the body axis. We will also mention the relevance of RF for medical conditions like hydrocephalus, scoliosis of the vertebral spine and possibly regeneration of the spinal cord.
Keywords: Reissner’s fiber; cerebrospinal fluid; chordates; neural tube; notochord; swimming behavior; vertebrates.
Copyright © 2021 Aboitiz and Montiel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

