Cadmium, an Environmental Contaminant, Exacerbates Alzheimer's Pathology in the Aged Mice's Brain
- PMID: 34248598
- PMCID: PMC8263901
- DOI: 10.3389/fnagi.2021.650930
Cadmium, an Environmental Contaminant, Exacerbates Alzheimer's Pathology in the Aged Mice's Brain
Abstract
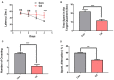
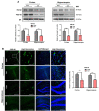
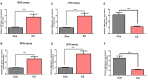
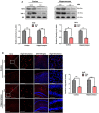
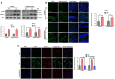
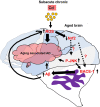
Cadmium (Cd) is an environmental contaminant, which is a potential risk factor in the progression of aging-associated neurodegenerative diseases. Herein, we have assessed the effects of chronic administration of Cd on cellular oxidative stress and its associated Alzheimer's disease (AD) pathologies in animal models. Two groups of mice were used, one group administered with saline and the other with Cd (1 mg/kg/day; intraperitoneally) for 3 months. After behavioral studies, molecular/biochemical (Immunoblotting, ELISAs, ROS, LPO, and GSH assays) and morphological analyses were performed. We observed an exacerbation of memory and synaptic deficits in chronic Cd-injected mice. Subacute and chronic Cd escalated reactive oxygen species (ROS), suppressed the master antioxidant enzymes, e.g., nuclear factor-erythroid 2-related factor 2 and heme oxygenase-1, and evoked the stress kinase phospho-c-Jun N-terminal kinase 1 signaling pathways, which may escalate AD pathologies possibly associated with amyloidogenic processes. These findings suggest the regulation of oxidative stress/ROS and its associated amyloid beta pathologies for targeting the Cd-exacerbated AD pathogenesis. In addition, these preclinical animal studies represent a paradigm for epidemiological studies of the human population exposed to chronic and subacute administration of Cd, suggesting avoiding environmental contaminants.
Keywords: Alzheimer’s disease; Cadmium; antioxidant genes Nrf-2/HO-1; neurodegeneration; reactive oxygen species.
Copyright © 2021 Ali, Khan, Alam, Ahmad, Ikram, Park, Lee and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ahmad A., Shah A. S., Badshah H., Kim M. J., Ali T., Yoon G. H., et al. (2016). Neuroprotection by vitamin C against ethanol-induced neuroinflammation associated neurodegeneration in the developing rat brain. CNS Neurol. Disord. Drug Targets 15, 360–370. 10.2174/1871527315666151110130139 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

