Ultrarapid Inflammation of the Olfactory Bulb After Spinal Cord Injury: Protective Effects of the Granulocyte Colony-Stimulating Factor on Early Neurodegeneration in the Brain
- PMID: 34248610
- PMCID: PMC8267925
- DOI: 10.3389/fnagi.2021.701702
Ultrarapid Inflammation of the Olfactory Bulb After Spinal Cord Injury: Protective Effects of the Granulocyte Colony-Stimulating Factor on Early Neurodegeneration in the Brain
Abstract
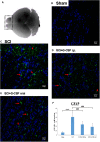
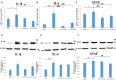
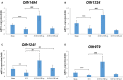
The correlation among olfactory dysfunction, spinal cord injury (SCI), subjective cognitive decline, and neurodegenerative dementia has been established. Impaired olfaction is considered a marker for neurodegeneration. Hence, there is a need to examine if SCI leads to olfactory dysfunction. In this study, the brain tissue of mice with spinal cord hemisection injury was subjected to microarray analysis. The mRNA expression levels of olfactory receptors in the brain began to decline at 8 h post-SCI. SCI promoted neuroinflammation, downregulated the expression of olfactory receptors, decreased the number of neural stem cells (NSCs), and inhibited the production of neurotrophic factors in the olfactory bulbs at 8 h post-SCI. In particular, the SCI group had upregulated mRNA and protein expression levels of glial fibrillary acidic protein (GFAP; a marker of astrocyte reactivation) and pro-inflammatory mediators [IL-1β, IL-6, and Nestin (marker of NSCs)] in the olfactory bulb compared to levels in the sham control group. The mRNA expression levels of olfactory receptors (Olfr1494, Olfr1324, Olfr1241, and Olfr979) and neurotrophic factors [brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), and nerve growth factor (NGF)] were downregulated in the olfactory bulb of the SCI group mice at 8 h post-SCI. The administration of granulocyte colony-stimulating factor (G-CSF) mitigated these SCI-induced pathological changes in the olfactory bulb at 8 h post-SCI. These results indicate that the olfactory bulb is vulnerable to environmental damage even if the lesion is located at sites distant from the brain, such as the spinal cord. Additionally, SCI initiated pathological processes, including inflammatory response, and impaired neurogenesis, at an early stage. The findings of this study will provide a basis for future studies on pathological mechanisms of early neurodegenerative diseases involving the olfactory bulb and enable early clinical drug intervention.
Keywords: granulocyte colony stimulating factor; neurodegenerative disease; neuroinflammation; olfactory bulb; olfactory dysfunction; spinal cord injury; subjective cognitive decline.
Copyright © 2021 Lin, Chiu and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Altinayar S., Oner S., Can S., Kizilay A., Kamisli S., Sarac K. (2014). Olfactory disfunction and its relation olfactory bulb volume in Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 18 3659–3664. - PubMed
-
- Benraiss A., Chmielnicki E., Lerner K., Roh D., Goldman S. A. (2001). Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J. Neurosci. 21 6718–6731. 10.1523/jneurosci.21-17-06718.2001 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

