Patient and Payer Preferences for Additional Value Criteria
- PMID: 34248638
- PMCID: PMC8263917
- DOI: 10.3389/fphar.2021.690021
Patient and Payer Preferences for Additional Value Criteria
Abstract
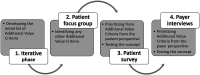
Background: Defining the value of healthcare is an elusive target, and depends heavily on the decision context and stakeholders involved. Cost-utility analysis and the quality-adjusted life year (QALY) have become the method and value definition of choice for traditional value judgements in coverage and pricing decisions. Other criteria that may influence value are often not measured and therefore omitted from value assessments, or are only used to qualitatively contextualize assessments. The objective of this study was to engage two key stakeholders; patients and payers to elicit and rank the importance of additional value criteria, potentially assessed in Multiple Criteria Decision Analysis (MCDA). Methods: This study consisted of a focus group with cancer patients (n = 7), including follow-up questions through an electronic survey, and in-depth phone interviews with payers (n = 5). Results: For payers, value equated either with criteria that provided tangible benefits (from their perspective) such as new treatment options that respond to serious unmet need. For patients, population-level value equated to options that would potentially benefit them in the future and the value of hope. However, these criteria were seen by payers as difficult to measure and incorporate into objective decision making. Limitations: The findings from this study are primarily limited due to generalizability. Due to the small sample size, it was outside the scope of this study to calculate a weight for each criterion that could be used as part of a quantitative MCDA. Conclusion: MCDA, with particular attention to qualitative aspects, is an avenue to incorporate these additional criteria into value assessments, as well as provide an opportunity for reflecting the patient's preferences in assessing the value of a treatment.
Keywords: Multiple Criteria Decision Analysis; health technology assessement; patient; payer; value.
Copyright © 2021 Jakab, Whittington, Franklin, Raiola, Campbell, Kaló and McQueen.
Conflict of interest statement
Author SR was employed by the company Real Endpoints, LLC. Author IJ and ZK were employed by Syreon Research Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

