Systemic Administration of Tempol Attenuates the Cardiorespiratory Depressant Effects of Fentanyl
- PMID: 34248639
- PMCID: PMC8260831
- DOI: 10.3389/fphar.2021.690407
Systemic Administration of Tempol Attenuates the Cardiorespiratory Depressant Effects of Fentanyl
Abstract
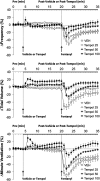
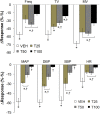
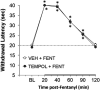
Fentanyl is a high-potency opioid receptor agonist that elicits profound analgesia and suppression of breathing in humans and animals. To date, there is limited evidence as to whether changes in oxidant stress are important factors in any of the actions of acutely administered fentanyl. This study determined whether the clinically approved superoxide dismutase mimetic, Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl), or a potent antioxidant, N-acetyl-L-cysteine methyl ester (L-NACme), modify the cardiorespiratory and analgesic actions of fentanyl. We examined whether the prior systemic injection of Tempol or L-NACme affects the cardiorespiratory and/or analgesic responses elicited by the subsequent injection of fentanyl in isoflurane-anesthetized and/or freely moving male Sprague-Dawley rats. Bolus injections of Tempol (25, 50 or 100 mg/kg, IV) elicited minor increases in frequency of breathing, tidal volume and minute ventilation. The ventilatory-depressant effects of fentanyl (5 μg/kg, IV) given 15 min later were dose-dependently inhibited by prior injections of Tempol. Tempol elicited dose-dependent and transient hypotension that had (except for the highest dose) resolved when fentanyl was injected. The hypotensive responses elicited by fentanyl were markedly blunted after Tempol pretreatment. The analgesic actions of fentanyl (25 μg/kg, IV) were not affected by Tempol (100 mg/kg, IV). L-NACme did not modify any of the effects of fentanyl. We conclude that prior administration of Tempol attenuates the cardiorespiratory actions of fentanyl without affecting the analgesic effects of this potent opioid. As such, Tempol may not directly affect opioid-receptors that elicit the effects of fentanyl. Whether, the effects of Tempol are solely due to alterations in oxidative stress is in doubt since the powerful antioxidant, L-NACme, did not affect fentanyl-induced suppression of breathing.
Keywords: cardiorespiratory depression; fentanyl; sprague-dawley rats; superoxide dismutase; tempol.
Copyright © 2021 Baby, Gruber, Discala, Puskovic, Jose, Cheng, Jenkins, Seckler and Lewis.
Conflict of interest statement
The authors, SB, RG, JD and VP were employed by the company Galleon Pharmaceuticals, Inc. The authors declare that this study received funding from Galleon Pharmaceuticals, Inc. The leadership of the company were not directly involved in this study as a commercial entity. Only the principal scientists of the company were involved in the study design, collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

