In vivo / in vitro Correlation of Pharmacokinetics of Gentamicin, Vancomycin, Teicoplanin and Doripenem in a Bovine Blood Hemodialysis Model
- PMID: 34248646
- PMCID: PMC8264131
- DOI: 10.3389/fphar.2021.702455
In vivo / in vitro Correlation of Pharmacokinetics of Gentamicin, Vancomycin, Teicoplanin and Doripenem in a Bovine Blood Hemodialysis Model
Abstract
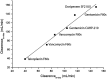
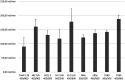
Background: Elimination of a drug during renal replacement therapy is not only dependent on flow rates, molecular size and protein binding, but is often influenced by difficult to predict drug membrane interactions. In vitro models allow for extensive profiling of drug clearance using a wide array of hemofilters and flow rates. We present a bovine blood based in vitro pharmacokinetic model for intermittent renal replacement therapy. Methods: Four different drugs were analyzed: gentamicin, doripenem, vancomicin and teicoplanin. The investigated drug was added to a bovine blood reservoir connected to a hemodialysis circuit. In total seven hemofilter models were analyzed using commonly employed flow rates. Pre-filter, post-filter and dialysate samples were drawn, plasmaseparated and analyzed using turbidimetric assays or HPLC. Protein binding of doripenem and vancomycin was measured in bovine plasma and compared to previously published values for human plasma. Results: Clearance values were heavily impacted by choice of membrane material and surface as well as by dialysis parameters such as blood flow rate. Gentamicin clearance ranged from a minimum of 90.12 ml/min in a Baxter CAHP-170 diacetate hemofilter up to a maximum of 187.90 ml/min in a Fresenius medical company Fx80 polysulfone model (blood flow rate 400 ml/min, dialysate flow rate 800 ml/min). Clearance of Gentamicin vs Vancomicin over the F80s hemofilter model using the same flow rates was 137.62 mL vs 103.25 ml/min. Doripenem clearance with the Fx80 was 141.25 ml/min. Conclusion: Clearance values corresponded very well to previously published data from clinical pharmacokinetic trials. In conjunction with in silico pharmacometric models. This model will allow precise dosing recommendations without the need of large scale clinical trials.
Keywords: antimicrobial agent; bovine blood; chronic hemodialysis; clearance; pharmacokinetics.
Copyright © 2021 Vossen, Pferschy, Milacek, Haidinger, Karolyi, Vass, Burgmann, Maier-Salamon, Wicha, Jäger, Zeitlinger, Stimpfl, Wittek and Thalhammer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bouman C. S. C., van Kan H. J. M., Koopmans R. P., Korevaar J. C., Schultz M. J., Vroom M. B. (2006). Discrepancies between Observed and Predicted Continuous Venovenous Hemofiltration Removal of Antimicrobial Agents in Critically Ill Patients and the Effects on Dosing. Intensive Care Med. 32, 2013–2019. 10.1007/s00134-006-0397-x - DOI - PubMed
LinkOut - more resources
Full Text Sources

