GLUT4 in Mouse Endometrial Epithelium: Roles in Embryonic Development and Implantation
- PMID: 34248664
- PMCID: PMC8267529
- DOI: 10.3389/fphys.2021.674924
GLUT4 in Mouse Endometrial Epithelium: Roles in Embryonic Development and Implantation
Abstract
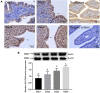
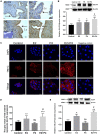
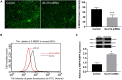
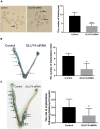
GLUT4 is involved in rapid glucose uptake among various kinds of cells to contribute to glucose homeostasis. Prior data have reported that aberrant glucose metabolism by GLUT4 dysfunction in the uterus could be responsible for infertility and increased miscarriage. However, the expression and precise functions of GLUT4 in the endometrium under physiological conditions remain unknown or controversial. In this study, we observed that GLUT4 exhibits a spatiotemporal expression in mouse uterus on pregnant days 1-4; its expression especially increased on pregnant day 4 during the window of implantation. We also determined that estrogen, in conjunction with progesterone, promotes the expression of GLUT4 in the endometrial epithelium in vivo or in vitro. GLUT4 is an important transporter that mediates glucose transport in endometrial epithelial cells (EECs) in vitro or in vivo. In vitro, glucose uptake decreased in mouse EECs when the cells were treated with GLUT4 small interfering RNA (siRNA). In vivo, the injection of GLUT4-siRNA into one side of the mouse uterine horns resulted in an increased glucose concentration in the uterine fluid on pregnant day 4, although it was still lower than in blood, and impaired endometrial receptivity by inhibiting pinopode formation and the expressions of leukemia inhibitory factor (LIF) and integrin ανβ3, finally affecting embryonic development and implantation. Overall, the obtained results indicate that GLUT4 in the endometrial epithelium affects embryo development by altering glucose concentration in the uterine fluid. It can also affect implantation by impairing endometrial receptivity due to dysfunction of GLUT4.
Keywords: embryonic development; endometrial epithelium; glucose transporter 4; implantation; uterine receptivity.
Copyright © 2021 Long, Wang, Yuan, Dai, Liao, Zhang, Zhang, Ma, Lei, Cui, Zhang, Nie and Yue.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Augustin R. (2010). The protein family of glucose transport facilitators: it’s not only about glucose after all. IUBMB Life 62 315–333. - PubMed
-
- Azkargorta M., Escobes I., Iloro I., Osinalde N., Corral B., Ibañez-Perez J., et al. (2018). Differential proteomic analysis of endometrial fluid suggests increased inflammation and impaired glucose metabolism in non-implantative IVF cycles and pinpoints PYGB as a putative implantation marker. Hum. Reprod. (Oxford, England) 33 1898–1906. 10.1093/humrep/dey274 - DOI - PubMed
-
- Baack M. L., Wang C., Hu S., Segar J. L., Norris A. W. (2014). Hyperglycemia induces embryopathy, even in the absence of systemic maternal diabetes: an in vivo test of the fuel mediated teratogenesis hypothesis. Reprod. Toxicol. (Elmsford, N.Y.) 46 129–136. 10.1016/j.reprotox.2014.03.013 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

