NRF2-Related Epigenetic Modifications in Cardiac and Vascular Complications of Diabetes Mellitus
- PMID: 34248833
- PMCID: PMC8269153
- DOI: 10.3389/fendo.2021.598005
NRF2-Related Epigenetic Modifications in Cardiac and Vascular Complications of Diabetes Mellitus
Abstract
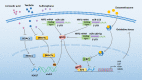
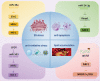
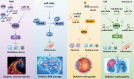
Diabetes mellitus (DM) is a highly prevalent chronic disease that is accompanied with serious complications, especially cardiac and vascular complications. Thus, there is an urgent need to identify new strategies to treat diabetic cardiac and vascular complications. Nuclear factor erythroid 2-related factor 2 (NRF2) has been verified as a crucial target for the prevention and treatment of diabetic complications. The function of NRF2 in the treatment of diabetic complications has been widely reported, but the role of NRF2-related epigenetic modifications remains unclear. The purpose of this review is to summarize the recent advances in targeting NRF2-related epigenetic modifications in the treatment of cardiac and vascular complications associated with DM. We also discuss agonists that could potentially regulate NRF2-associated epigenetic mechanisms. This review provides a better understanding of strategies to target NRF2 to protect against DM-related cardiac and vascular complications.
Keywords: NRF2; NRF2 activators; diabetic cardiac complication; diabetic vascular complication; epigenetic modifications.
Copyright © 2021 Wang, Xiao, Wang, Wang, Zhang, Guo, Tang and Gu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewers ZJ & SZ declared a shared affiliation with one of the authors, SW, to the handling editor at time of review.
Figures
Similar articles
-
The Role of Fibroblast Growth Factor 21 in Diabetic Cardiovascular Complications and Related Epigenetic Mechanisms.Front Endocrinol (Lausanne). 2021 Jul 19;12:598008. doi: 10.3389/fendo.2021.598008. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34349728 Free PMC article. Review.
-
Context-Dependent Regulation of Nrf2/ARE Axis on Vascular Cell Function during Hyperglycemic Condition.Curr Diabetes Rev. 2020;16(8):797-806. doi: 10.2174/1573399816666200130094512. Curr Diabetes Rev. 2020. PMID: 32000646 Review.
-
Current Status and Challenges of NRF2 as a Potential Therapeutic Target for Diabetic Cardiomyopathy.Int Heart J. 2019 May 30;60(3):512-520. doi: 10.1536/ihj.18-476. Epub 2019 Apr 10. Int Heart J. 2019. PMID: 30971629 Review.
-
The pivotal role of nuclear factor erythroid 2-related factor 2 in diabetes-induced endothelial dysfunction.Pharmacol Res. 2020 Mar;153:104601. doi: 10.1016/j.phrs.2019.104601. Epub 2019 Dec 12. Pharmacol Res. 2020. PMID: 31838079 Review.
-
Comprehensive overview of Nrf2-related epigenetic regulations involved in ischemia-reperfusion injury.Theranostics. 2022 Sep 11;12(15):6626-6645. doi: 10.7150/thno.77243. eCollection 2022. Theranostics. 2022. PMID: 36185600 Free PMC article. Review.
Cited by
-
Oxidative Stress Induced by Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) Dysfunction Aggravates Chronic Inflammation Through the NAD+/SIRT3 Axis and Promotes Renal Injury in Diabetes.Antioxidants (Basel). 2025 Feb 25;14(3):267. doi: 10.3390/antiox14030267. Antioxidants (Basel). 2025. PMID: 40227196 Free PMC article. Review.
-
Targeting epigenetic and post-translational modifications of NRF2: key regulatory factors in disease treatment.Cell Death Discov. 2025 Apr 21;11(1):189. doi: 10.1038/s41420-025-02491-z. Cell Death Discov. 2025. PMID: 40258841 Free PMC article. Review.
-
RETRACTED: The Emerging Role of Epigenetics in Metabolism and Endocrinology.Biology (Basel). 2023 Feb 6;12(2):256. doi: 10.3390/biology12020256. Biology (Basel). 2023. Retraction in: Biology (Basel). 2025 Jun 16;14(6):705. doi: 10.3390/biology14060705. PMID: 36829533 Free PMC article. Retracted. Review.
-
Risk factors for ninety-day readmission following cervical surgery: a meta-analysis.J Orthop Surg Res. 2022 Nov 3;17(1):477. doi: 10.1186/s13018-022-03377-x. J Orthop Surg Res. 2022. PMID: 36329494 Free PMC article.
-
Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases.Molecules. 2022 Jan 19;27(3):624. doi: 10.3390/molecules27030624. Molecules. 2022. PMID: 35163897 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

