Dark Septate Endophytes Isolated From Wild Licorice Roots Grown in the Desert Regions of Northwest China Enhance the Growth of Host Plants Under Water Deficit Stress
- PMID: 34248857
- PMCID: PMC8260703
- DOI: 10.3389/fmicb.2021.522449
Dark Septate Endophytes Isolated From Wild Licorice Roots Grown in the Desert Regions of Northwest China Enhance the Growth of Host Plants Under Water Deficit Stress
Abstract
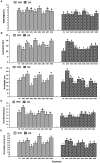
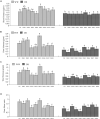
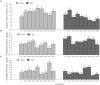
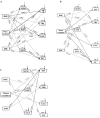
This study aimed to explore dark septate endophytes (DSE) that may improve the cultivation of medicinal plants in arid ecosystems. We isolated and identified eight DSE species (Acremonium nepalense, Acrocalymma vagum, Alternaria chartarum, Alternaria chlamydospora, Alternaria longissima, Darksidea alpha, Paraphoma chrysanthemicola, and Preussia terricola) colonizing the roots of wild licorice (Glycyrrhiza uralensis) in the desert areas of northwest China. Moreover, we investigated the osmotic stress tolerance of the DSE using pure culture, along with the performance of licorice plants inoculated with the DSE under drought stress in a growth chamber, respectively. Here, five species were first reported in desert habitats. The osmotic-stress tolerance of DSE species was highly variable, A. chlamydospora and P. terricola increased the total biomass and root biomass of the host plant. All DSE except A. vagum and P. chrysanthemicola increased the glycyrrhizic acid content; all DSE except A. chartarum increased the glycyrrhizin content under drought stress. DSE × watering regimen improved the glycyrrhizic acid content, soil organic matter, and available nitrogen. Structural equation model analysis showed that DSE × watering regimen positively affected soil organic matter, and total biomass, root length, glycyrrhizic acid, and glycyrrhizin (Shapotou site); and positively affected soil organic matter, available phosphorus, and glycyrrhizin (Minqin site); and positively affected the root length (Anxi site). DSE from the Shapotou site accounted for 8.0, 13.0, and 11.3% of the variations in total biomass, root biomass, and active ingredient content; DSE from the Minqin site accounted for 6.6 and 8.3% of the variations in total biomass and root biomass; DSE from the Anxi site accounted for 4.2 and 10.7% of the variations in total biomass and root biomass. DSE × watering regimen displayed a general synergistic effect on plant growth and active ingredient contents. These findings suggested that the DSE-plant interactions were affected by both DSE species and DSE originating habitats. As A. chlamydospora and P. terricola positively affected the total biomass, root biomass, and active ingredient content of host plants under drought stress, they may have important uses as promoters for the cultivation of licorice in dryland agriculture.
Keywords: dark septate endophytes; desert ecosystem; drought stress; inoculation; licorice.
Copyright © 2021 He, Wang, Hou and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Plant Growth and Soil Microbial Impacts of Enhancing Licorice With Inoculating Dark Septate Endophytes Under Drought Stress.Front Microbiol. 2019 Oct 9;10:2277. doi: 10.3389/fmicb.2019.02277. eCollection 2019. Front Microbiol. 2019. PMID: 31649632 Free PMC article.
-
Characterization of Dark Septate Endophytic Fungi and Improve the Performance of Liquorice Under Organic Residue Treatment.Front Microbiol. 2019 Jun 18;10:1364. doi: 10.3389/fmicb.2019.01364. eCollection 2019. Front Microbiol. 2019. PMID: 31275282 Free PMC article.
-
Plant performance of enhancing licorice with dual inoculating dark septate endophytes and Trichoderma viride mediated via effects on root development.BMC Plant Biol. 2020 Jul 9;20(1):325. doi: 10.1186/s12870-020-02535-9. BMC Plant Biol. 2020. PMID: 32646473 Free PMC article.
-
Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: resilience to biotic and abiotic stresses.World J Microbiol Biotechnol. 2022 Mar 25;38(5):79. doi: 10.1007/s11274-022-03264-x. World J Microbiol Biotechnol. 2022. PMID: 35332399 Review.
-
Dark septate endophytes: mutualism from by-products?Trends Plant Sci. 2022 Mar;27(3):247-254. doi: 10.1016/j.tplants.2021.10.001. Epub 2021 Oct 27. Trends Plant Sci. 2022. PMID: 34756535 Review.
Cited by
-
Deciphering the roles of bacterial and fungal communities in the formation and quality of agarwood.Stress Biol. 2024 Sep 20;4(1):40. doi: 10.1007/s44154-024-00179-5. Stress Biol. 2024. PMID: 39302547 Free PMC article.
-
Genotype and environment factors driven licorice growth and rhizospheric soil fungal community changes.Front Microbiol. 2023 Nov 23;14:1308412. doi: 10.3389/fmicb.2023.1308412. eCollection 2023. Front Microbiol. 2023. PMID: 38075860 Free PMC article.
-
A Dark Septate Endophyte Improves Cadmium Tolerance of Maize by Modifying Root Morphology and Promoting Cadmium Binding to the Cell Wall and Phosphate.J Fungi (Basel). 2023 Apr 29;9(5):531. doi: 10.3390/jof9050531. J Fungi (Basel). 2023. PMID: 37233243 Free PMC article.
-
Construing the resilience to osmotic stress using endophytic fungus in maize (Zea mays L.).Plant Mol Biol. 2025 Jan 17;115(1):22. doi: 10.1007/s11103-025-01550-4. Plant Mol Biol. 2025. PMID: 39821570
-
Species identity and combinations differ in their overall benefits to Astragalus adsurgens plants inoculated with single or multiple endophytic fungi under drought conditions.Front Plant Sci. 2022 Sep 7;13:933738. doi: 10.3389/fpls.2022.933738. eCollection 2022. Front Plant Sci. 2022. PMID: 36160950 Free PMC article.
References
-
- Aghai M. M., Khan Z., Joseph M. R., Stoda A. M., Sher A. W., Ettl G. J., et al. (2019). The effect of microbial endophyte consortia on Pseudotsuga menziesii and Thuja plicata survival, growth, and physiology across edaphic gradients. Front. Microbiol. 10:1353. 10.3389/fmicb.2019.01353 - DOI - PMC - PubMed
-
- Alberton O., Kuyper T. W., Summerbell R. C. (2010). Dark septate root endophytic fungi increase growth of Scots pine seedlings under elevated CO2 through enhanced nitrogen use efficiency. Plant Soil 328 459–470. 10.1007/s11104-009-0125-8 - DOI
-
- Al-Hosni K., Shahzad R., Khan A. L., Imran Q. M., Al Harrasi A., Al Rawahi A., et al. (2018). Preussia sp. BSL-10 producing nitric oxide, gibberellins, and indole acetic acid and improving rice plant growth. J. Plant Interact. 13 112–118. 10.1080/17429145.2018.1432773 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

