Transfer of PBMC From SSc Patients Induces Autoantibodies and Systemic Inflammation in Rag2-/-/IL2rg-/- Mice
- PMID: 34248959
- PMCID: PMC8261241
- DOI: 10.3389/fimmu.2021.677970
Transfer of PBMC From SSc Patients Induces Autoantibodies and Systemic Inflammation in Rag2-/-/IL2rg-/- Mice
Abstract
Objective: The contribution of sustained autologous autoantibody production by B cells to the pathogenesis of systemic sclerosis (SSc) and granulomatosis with polyangiitis (GPA) is not fully understood. To investigate this, a humanized mouse model was generated by transferring patient-derived peripheral blood mononuclear cells (PBMC) into immunocompromised mice.
Methods: PBMC derived from patients with SSc and GPA as well as healthy controls (HD) were isolated, characterized by flow cytometry, and infused into Rag2-/-/IL2rg-/- mice. In addition, PBMC from SSc patients treated with rituximab were transferred into mice. Twelve weeks later, human autoantibodies were determined in blood of the recipient mice and affected tissues were analyzed for pathological changes by histology and immunohistochemistry.
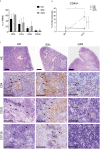
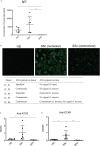
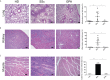
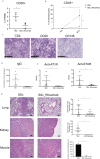
Results: Mice engrafted with PBMC derived from SSc patients developed autoantibodies such as antinuclear antibodies (ANA) mimicking the pattern of the respective donors. Moreover, cellular infiltrates dominated by B cells were observed in lung, kidney and muscles of the recipient mice. By contrast, PBMC derived from HD or GPA patients survived in recipient mice after transfer, but neither human autoantibodies nor inflammatory infiltrates in tissues were detected. Furthermore, these pathological changes were absent in mice transferred with PBMC from rituximab-treated SSc patients.
Conclusion: This humanized mouse model is indicative for cross-reactivity of human lymphocytes to murine autoantigens and argues for a pivotal role of B cells as well as of sustained autoimmunity in the pathogenesis of SSc. It provides a powerful tool to study interstitial lung disease and so far, under-recognized disease manifestations such as myositis and interstitial nephritis.
Keywords: B cells; autoantibodies; autoimmune diseases; granulomatosis with polyangiitis; humanized mouse model; peripheral blood mononuclear cells; systemic inflammation; systemic sclerosis.
Copyright © 2021 Yue, Petersen, Shu, Kasper, Magatsin, Ahmadi, Yin, Wax, Wang, Heidecke, Lamprecht, Müller, Yu and Riemekasten.
Conflict of interest statement
HH is the owner and GR is an advisor of the company CellTrend, Germany, which produced the membrane extracts and the tests for the detection of anti-AT1R antibodies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

