A Flow Cytometric Assay to Detect Functional Ganglionic Acetylcholine Receptor Antibodies by Immunomodulation in Autoimmune Autonomic Ganglionopathy
- PMID: 34249013
- PMCID: PMC8261233
- DOI: 10.3389/fimmu.2021.705292
A Flow Cytometric Assay to Detect Functional Ganglionic Acetylcholine Receptor Antibodies by Immunomodulation in Autoimmune Autonomic Ganglionopathy
Abstract
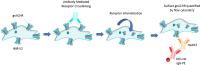
Autoimmune Autonomic Ganglionopathy (AAG) is an uncommon immune-mediated neurological disease that results in failure of autonomic function and is associated with autoantibodies directed against the ganglionic acetylcholine receptor (gnACHR). The antibodies are routinely detected by immunoprecipitation assays, such as radioimmunoassays (RIA), although these assays do not detect all patients with AAG and may yield false positive results. Autoantibodies against the gnACHR exert pathology by receptor modulation. Flow cytometric analysis is able to determine if this has occurred, in contrast to the assays in current use that rely on immunoprecipitation. Here, we describe the first high-throughput, non-radioactive flow cytometric assay to determine autoantibody mediated gnACHR immunomodulation. Previously identified gnACHR antibody seronegative and seropositive sera samples (RIA confirmed) were blinded and obtained from the Oxford Neuroimmunology group along with samples collected locally from patients with or without AAG. All samples were assessed for the ability to cause gnACHR immunomodulation utilizing the prototypical gnACHR expressing cell line, IMR-32. Decision limits were calculated from healthy controls, and Receiver Operating Characteristic (ROC) curves were constructed after unblinding all samples. One hundred and ninety serum samples were analyzed; all 182 expected negative samples (from healthy controls, autonomic disorders not thought to be AAG, other neurological disorders without autonomic dysfunction and patients with Systemic Lupus Erythematosus) were negative for immunomodulation (<18%), as were the RIA negative AAG and unconfirmed AAG samples. All RIA positive samples displayed significant immunomodulation. There were no false positive or negative samples. There was perfect qualitative concordance as compared to RIA, with an Area Under ROC of 1. Detection of Immunomodulation by flow cytometry for the identification of gnACHR autoantibodies offers excellent concordance with the gnACHR antibody RIA, and overcomes many of the shortcomings of immunoprecipitation assays by directly measuring the pathological effects of these autoantibodies at the cellular level. Further work is needed to determine the correlation between the degree of immunomodulation and disease severity.
Keywords: autoimmune autonomic ganglionopathy; diagnostic test; flow cytometry—methods; immunoassay; neuroimmunology.
Copyright © 2021 Urriola, Spies, Blazek, Lang and Adelstein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Nakane S, Higuchi O, Koka M, Kanda T, Murata K, Suzuki T, et al. Clinical Features of Autoimmune Autonomic Ganglionopathy and the Detection of Subunit-Specific Autoantibodies to the Ganglionic Acetylcholine Receptor in Japanese Patients. PloS One (2015) 10:e0118312. 10.1371/journal.pone.0118312 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

