Metabolic Control of Smoldering Neuroinflammation
- PMID: 34249016
- PMCID: PMC8262770
- DOI: 10.3389/fimmu.2021.705920
Metabolic Control of Smoldering Neuroinflammation
Abstract
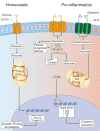
Compelling evidence exists that patients with chronic neurological conditions, which includes progressive multiple sclerosis, display pathological changes in neural metabolism and mitochondrial function. However, it is unknown if a similar degree of metabolic dysfunction occurs also in non-neural cells in the central nervous system. Specifically, it remains to be clarified (i) the full extent of metabolic changes in tissue-resident microglia and infiltrating macrophages after prolonged neuroinflammation (e.g., at the level of chronic active lesions), and (ii) whether these alterations underlie a unique pathogenic phenotype that is amenable for therapeutic targeting. Herein, we discuss how cell metabolism and mitochondrial function govern the function of chronic active microglia and macrophages brain infiltrates and identify new metabolic targets for therapeutic approaches aimed at reducing smoldering neuroinflammation.
Keywords: immunometabolism; macrophages; metabolism; microglia; mitochondria; progressive multiple sclerosis; smoldering inflammation.
Copyright © 2021 Peruzzotti-Jametti, Willis, Hamel, Krzak and Pluchino.
Conflict of interest statement
SP is co-founder, CSO, and shareholder (>5%) of CITC Ltd. and iSTEM Therapeutics and co-founder and Non-Executive Director at Asitia Therapeutics; LP-J is a shareholder of CITC Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

