Alternative Oxidase Inhibition Impairs Tobacco Root Development and Root Hair Formation
- PMID: 34249036
- PMCID: PMC8264555
- DOI: 10.3389/fpls.2021.664792
Alternative Oxidase Inhibition Impairs Tobacco Root Development and Root Hair Formation
Abstract
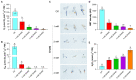
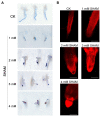
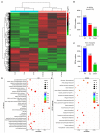
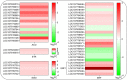
Alternative oxidase (AOX) is the terminal oxidase of the mitochondrial respiratory electron transport chain in plant cells and is critical for the balance of mitochondrial hemostasis. In this study, the effect of inhibition of AOX with different concentrations of salicylhydroxamic acid (SHAM) on the tobacco root development was investigated. We show here that AOX inhibition significantly impaired the development of the main root and root hair formation of tobacco. The length of the main root of SHAM-treated tobacco was significantly shorter than that of the control, and no root hairs were formed after treatment with a concentration of 1 mM SHAM or more. The transcriptome analysis showed that AOX inhibition by 1 mM SHAM involved in the regulation of gene expression related to root architecture. A total of 5,855 differentially expressed genes (DEGs) were obtained by comparing SHAM-treated roots with control. Of these, the gene expression related to auxin biosynthesis and perception were significantly downregulated by 1 mM SHAM. Similarly, genes related to cell wall loosening, cell cycle, and root meristem growth factor 1 (RGF1) also showed downregulation on SHAM treatment. Moreover, combined with the results of physiological measurements, the transcriptome analysis demonstrated that AOX inhibition resulted in excessive accumulation of reactive oxygen species in roots, which further induced oxidative damage and cell apoptosis. It is worth noting that when indoleacetic acid (20 nM) and dimethylthiourea (10 mM) were added to the medium containing SHAM, the defects of tobacco root development were alleviated, but to a limited extent. Together, these findings indicated that AOX-mediated respiratory pathway plays a crucial role in the tobacco root development, including root hair formation.
Keywords: alternative oxidase; auxin; respiration; root architecture; tobacco.
Copyright © 2021 Liu, Yu, Peng, Geng and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Chandrakar V., Keshavkant S. (2018). Nitric oxide and dimethylthiourea up-regulates pyrroline-5-carboxylate synthetase expression to improve arsenic tolerance in Glycine max L. Environ. Prog. Sustain. Energy 38, 402–409. 10.1002/ep.12978 - DOI
LinkOut - more resources
Full Text Sources

