Evaluation of the short-term efficacy of local analgesic (lidocaine) and opioid analgesic (sufentanil) on patients with centrally mediated abdominal pain syndrome: a randomized controlled trial
- PMID: 34249145
- PMCID: PMC8237217
- DOI: 10.1177/17562848211021783
Evaluation of the short-term efficacy of local analgesic (lidocaine) and opioid analgesic (sufentanil) on patients with centrally mediated abdominal pain syndrome: a randomized controlled trial
Abstract
Background: Centrally mediated abdominal pain syndrome (CAPS) is characterized by continuous or frequently recurring abdominal pain and can result in functional loss across several life domains. The efficacy of the present management methods has not been established yet. We performed a prospective randomized controlled trial to explore the short-term efficacy of local analgesic (lidocaine) and opioid analgesic (sufentanil) in patients with CAPS.
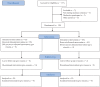
Methods: We consecutively enrolled 130 patients who met the Rome IV CAPS criteria and divided them into the sufentanil + lidocaine (S + L) group and sufentanil (S) group. Patients completed the pain rating scales, including the numeric rating scale (NRS) and verbal rating scale (VRS), 60 min before colonoscopy. All the patients were initially administered sufentanil. In the S + L group, we sprayed a 5 ml solution of lidocaine on the surface of ascending, transverse, descending, and sigmoid colon during colonoscope withdrawal, while 5 ml saline was sprayed in the S group. Follow up was performed 1 day, 3 days, 1 week, 2 weeks, 1 month, and 3 months after colonoscopy, to complete the pain scaling.
Results: A comparison of the NRS and VRS showed that there were no significant differences between the S + L and S groups and within each group (p > 0.05).
Conclusions: Local analgesic lidocaine and opioid analgesic sufentanil showed negative efficacy during short-term observation. The opioid receptor blocker sufentanil did not worsen symptoms in patients with CAPS after colonoscopy under general anesthesia in the short term.[chictr.org.cn, Chinese Clinical Trial Identifier, ChiCTR-IOR-16008187].
Keywords: centrally mediated abdominal pain syndrome; lidocaine; sufentanil; treatment.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Intradermal sufentanil does not improve lidocaine-induced local anesthesia.Can J Anaesth. 2003 Feb;50(2):153-8. doi: 10.1007/BF03017848. Can J Anaesth. 2003. PMID: 12560306 Clinical Trial.
-
Patient-controlled subcutaneous analgesia using sufentainil or morphine in home care treatment in patients with stage III-IV cancer: A multi-center randomized controlled clinical trial.Cancer Med. 2020 Aug;9(15):5345-5352. doi: 10.1002/cam4.3194. Epub 2020 Jun 4. Cancer Med. 2020. PMID: 32500675 Free PMC article. Clinical Trial.
-
Analgesic effects of intrathecal sufentanil added to lidocaine 5% in elective cesarean section.Acta Med Iran. 2010 Nov-Dec;48(6):380-4. Acta Med Iran. 2010. PMID: 21287477 Clinical Trial.
-
50% efficacy dose of intravenous lidocaine in supressing sufentanil-induced cough in children: a randomised controlled trial.BMC Anesthesiol. 2024 Apr 19;24(1):149. doi: 10.1186/s12871-024-02541-6. BMC Anesthesiol. 2024. PMID: 38641778 Free PMC article. Clinical Trial.
-
Prospective investigation of intravenous patient-controlled analgesia with hydromorphone or sufentanil: impact on mood, opioid adverse effects, and recovery.BMC Anesthesiol. 2018 Apr 10;18(1):37. doi: 10.1186/s12871-018-0500-1. BMC Anesthesiol. 2018. PMID: 29636011 Free PMC article. Clinical Trial.
Cited by
-
Comparison of pre-treatment with different diluted sufentanil in reducing propofol injection pain in gastrointestinal endoscopy: A randomized controlled study.PLoS One. 2025 May 29;20(5):e0325113. doi: 10.1371/journal.pone.0325113. eCollection 2025. PLoS One. 2025. PMID: 40440342 Free PMC article. Clinical Trial.
References
-
- Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology 2016; 150: 1257–1261. - PubMed
-
- Cremon C, Carini G, Wang B, et al.. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol 2011; 106: 1290–1298. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous