Role of the miR-133a-5p/FBXO6 axis in the regulation of intervertebral disc degeneration
- PMID: 34249610
- PMCID: PMC8233105
- DOI: 10.1016/j.jot.2021.05.004
Role of the miR-133a-5p/FBXO6 axis in the regulation of intervertebral disc degeneration
Abstract
Objective: Low back pain is a leading cause of disabilities worldwide, and intervertebral disc degeneration (IVDD)-related disorders have been recognised as one of the main contributors. Nevertheless, the underlying mechanism has not yet been fully understood. The aim of this study was to investigate the role of the miR-133a-5p/FBXO6 axis in the regulation of IVDD.
Methods: RT-qPCR, WB and IHC were performed to assess the expression of FBXO6 in human IVD tissues. Nucleus pulposus (NP) cells were treated with IL-1β to induce IVDD cellular model. Silence of FBXO6 was achieved using specific siRNAs. CCK-8 assay, flow cytometry, TUNEL assay, RT-qPCR and WB were used to evaluate the role and mechanism of FBXO6 in the process of IVDD. Online tools, GSE datasets and RT-qPCR were used to search the candidate miRNAs targeting FBXO6. The direct binding sites between FBXO6 and miR-133a-5p were further verified by a dual luciferase assay. RT-qPCR, WB and rescue experiments were conducted to identify the regulatory function of miR-133a-5p on the expression of aggrecan, collagen Ⅱ, MMP3, ADAMTS5, IL-6 and COX2. In addition, the role of the NF-κB pathway in regulating miR-133a-5p was studied using lentiviral shRNA, WB and RT-qPCR.
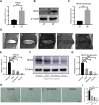
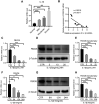
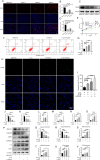
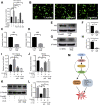
Results: Results showed that FBXO6 mainly expressed in the NP tissue of IVD and the expression of FBXO6 decreased with the process of IVDD as well as under IL-1β stimulation. The silence of FBXO6 led to the decreased expression of aggrecan and collagen Ⅱ and the increased expression of MMP3, ADAMTS5, IL-6 and COX2, which further induced the degeneration of NP cells. The bioinformatic analysis showed that miR-133a-5p was the candidate miRNA targeting FBXO6. miR-133a-5p was upregulated in IVDD tissues and significantly inhibited the expression of FBXO6. The inhibition of miR-133a-5p ameliorated the acceleration of IVDD induced by the silence of FBXO6 in vitro. Moreover, it was demonstrated that IL-1β regulated the expression of the miR-133a-5p/FBXO6 axis via the NF-κB pathway in NP cells.
Conclusion: miR-133a-5p was upregulated by IL-1β to aggravate intervertebral disc degeneration via sponging FBXO6. Inhibiting miR-133a-5p expression or rescuing FBXO6 expression may be promising strategies for the treatment of IVDD.
The translational potential of this article: This study suggests that the miR-133a-5p/FBXO6 axis could regulate NP cells proliferation, apoptosis, synthesis and degradation of extracellular matrix, which provides a promising therapeutic target and strategy for the treatment of IVDD.
Keywords: FBXO6; Interleukin (IL)-1β; Intervertebral disc; Nucleus pulposus cells; miR-133a-5p.
© 2021 The Authors.
Conflict of interest statement
The authors have no conflicts of interest to disclose in relation to this article.
Figures


Similar articles
-
Mechanism of KMT2D-mediated epigenetic modification in IL-1β-induced nucleus pulposus cell degeneration.Histol Histopathol. 2025 May;40(5):733-743. doi: 10.14670/HH-18-813. Epub 2024 Sep 12. Histol Histopathol. 2025. PMID: 39380528
-
Ginsenoside Rg1 relieves rat intervertebral disc degeneration and inhibits IL-1β-induced nucleus pulposus cell apoptosis and inflammation via NF-κB signaling pathway.In Vitro Cell Dev Biol Anim. 2024 Mar;60(3):287-299. doi: 10.1007/s11626-024-00883-6. Epub 2024 Mar 14. In Vitro Cell Dev Biol Anim. 2024. PMID: 38485818 Free PMC article.
-
FOXO3-Activated circFGFBP1 Inhibits Extracellular Matrix Degradation and Nucleus Pulposus Cell Death via miR-9-5p/BMP2 Axis in Intervertebral Disc Degeneration In Vivo and In Vitro.Pharmaceuticals (Basel). 2023 Mar 22;16(3):473. doi: 10.3390/ph16030473. Pharmaceuticals (Basel). 2023. PMID: 36986573 Free PMC article.
-
Role of oxidative stress in mitochondrial dysfunction and their implications in intervertebral disc degeneration: Mechanisms and therapeutic strategies.J Orthop Translat. 2024 Oct 16;49:181-206. doi: 10.1016/j.jot.2024.08.016. eCollection 2024 Nov. J Orthop Translat. 2024. PMID: 39483126 Free PMC article. Review.
-
MicroRNA-targeting nanomedicines for the treatment of intervertebral disc degeneration.Adv Drug Deliv Rev. 2024 Apr;207:115214. doi: 10.1016/j.addr.2024.115214. Epub 2024 Feb 22. Adv Drug Deliv Rev. 2024. PMID: 38395361 Review.
Cited by
-
Single-cell RNA sequencing reveals resident progenitor and vascularization-associated cell subpopulations in rat annulus fibrosus.J Orthop Translat. 2022 Dec 8;38:256-267. doi: 10.1016/j.jot.2022.11.004. eCollection 2023 Jan. J Orthop Translat. 2022. PMID: 36568849 Free PMC article.
-
Understanding Intervertebral Disc Degeneration: Background Factors and the Role of Initial Injury.Biomedicines. 2023 Oct 6;11(10):2714. doi: 10.3390/biomedicines11102714. Biomedicines. 2023. PMID: 37893088 Free PMC article. Review.
-
A diagnostic signatures for intervertebral disc degeneration using TNFAIP6 and COL6A2 based on single-cell RNA-seq and bulk RNA-seq analyses.Ann Med. 2025 Dec;57(1):2443568. doi: 10.1080/07853890.2024.2443568. Epub 2024 Dec 20. Ann Med. 2025. PMID: 39704340 Free PMC article.
-
Mechanism of KMT2D-mediated epigenetic modification in IL-1β-induced nucleus pulposus cell degeneration.Histol Histopathol. 2025 May;40(5):733-743. doi: 10.14670/HH-18-813. Epub 2024 Sep 12. Histol Histopathol. 2025. PMID: 39380528
-
Regulated cell death: Implications for intervertebral disc degeneration and therapy.J Orthop Translat. 2022 Nov 5;37:163-172. doi: 10.1016/j.jot.2022.10.009. eCollection 2022 Nov. J Orthop Translat. 2022. PMID: 36380883 Free PMC article. Review.
References
-
- Buchbinder R., van Tulder M., Oberg B., Costa L.M., Woolf A., Schoene M. Low back pain: a call for action. Lancet. 2018;391(10137):2384–2388. - PubMed
-
- Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., Abdulkader R.S. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. - PMC - PubMed
-
- Vos T., Barber R.M., Bell B., Bertozzi-Villa A., Biryukov S., Bolliger I. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. - PMC - PubMed
-
- Wang H., Liu H., Zheng Z.M., Zhang K.B., Wang T.P., Sribastav S.S. Role of death receptor, mitochondrial and endoplasmic reticulum pathways in different stages of degenerative human lumbar disc. Apoptosis. 2011;16(10):990–1003. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

