The Effects of Environmental Adversities on Human Neocortical Neurogenesis Modeled in Brain Organoids
- PMID: 34250020
- PMCID: PMC8264783
- DOI: 10.3389/fmolb.2021.686410
The Effects of Environmental Adversities on Human Neocortical Neurogenesis Modeled in Brain Organoids
Abstract
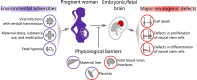
Over the past decades, a growing body of evidence has demonstrated the impact of prenatal environmental adversity on the development of the human embryonic and fetal brain. Prenatal environmental adversity includes infectious agents, medication, and substances of use as well as inherently maternal factors, such as diabetes and stress. These adversities may cause long-lasting effects if occurring in sensitive time windows and, therefore, have high clinical relevance. However, our knowledge of their influence on specific cellular and molecular processes of in utero brain development remains scarce. This gap of knowledge can be partially explained by the restricted experimental access to the human embryonic and fetal brain and limited recapitulation of human-specific neurodevelopmental events in model organisms. In the past years, novel 3D human stem cell-based in vitro modeling systems, so-called brain organoids, have proven their applicability for modeling early events of human brain development in health and disease. Since their emergence, brain organoids have been successfully employed to study molecular mechanisms of Zika and Herpes simplex virus-associated microcephaly, as well as more subtle events happening upon maternal alcohol and nicotine consumption. These studies converge on pathological mechanisms targeting neural stem cells. In this review, we discuss how brain organoids have recently revealed commonalities and differences in the effects of environmental adversities on human neurogenesis. We highlight both the breakthroughs in understanding the molecular consequences of environmental exposures achieved using organoids as well as the on-going challenges in the field related to variability in protocols and a lack of benchmarking, which make cross-study comparisons difficult.
Keywords: brain organoid; corticogenesis; environmental programming; neural stem/progenitor cells; neurogenesis.
Copyright © 2021 Sarieva and Mayer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Stem cell-based region-specific brain organoids: Novel models to understand neurodevelopmental defects.Birth Defects Res. 2022 Oct 1;114(16):1003-1013. doi: 10.1002/bdr2.2004. Epub 2022 Mar 25. Birth Defects Res. 2022. PMID: 35332709 Review.
-
Neurodevelopmental signatures of narcotic and neuropsychiatric risk factors in 3D human-derived forebrain organoids.Mol Psychiatry. 2021 Dec;26(12):7760-7783. doi: 10.1038/s41380-021-01189-9. Epub 2021 Jun 22. Mol Psychiatry. 2021. PMID: 34158620 Free PMC article.
-
Benchmarking brain organoid recapitulation of fetal corticogenesis.Transl Psychiatry. 2022 Dec 20;12(1):520. doi: 10.1038/s41398-022-02279-0. Transl Psychiatry. 2022. PMID: 36539399 Free PMC article.
-
What Makes Organoids Good Models of Human Neurogenesis?Front Neurosci. 2022 Apr 14;16:872794. doi: 10.3389/fnins.2022.872794. eCollection 2022. Front Neurosci. 2022. PMID: 35495031 Free PMC article.
-
Human Brain Organoids to Decode Mechanisms of Microcephaly.Front Cell Neurosci. 2020 May 8;14:115. doi: 10.3389/fncel.2020.00115. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32457578 Free PMC article. Review.
Cited by
-
Prenatal nicotine alters development of the laterodorsal tegmentum: Possible role for attention-deficit/hyperactivity disorder and drug dependence.World J Psychiatry. 2022 Feb 19;12(2):212-235. doi: 10.5498/wjp.v12.i2.212. eCollection 2022 Feb 19. World J Psychiatry. 2022. PMID: 35317337 Free PMC article. Review.
-
Are the Organoid Models an Invaluable Contribution to ZIKA Virus Research?Pathogens. 2021 Sep 24;10(10):1233. doi: 10.3390/pathogens10101233. Pathogens. 2021. PMID: 34684182 Free PMC article. Review.
-
Prenatal Hypoxia Affects Nicotine Consumption and Withdrawal in Adult Rats via Impairment of the Glutamate System in the Brain.Mol Neurobiol. 2022 Jul;59(7):4550-4561. doi: 10.1007/s12035-022-02866-8. Epub 2022 May 17. Mol Neurobiol. 2022. PMID: 35581520
-
Prenatal Drugs and Their Effects on the Developing Brain: Insights From Three-Dimensional Human Organoids.Front Neurosci. 2022 Mar 25;16:848648. doi: 10.3389/fnins.2022.848648. eCollection 2022. Front Neurosci. 2022. PMID: 35401083 Free PMC article. Review.
-
Environmental adversity, endoplasmic reticulum stress, and neurogenesis.Neurotoxicology. 2025 Jul;109:32-45. doi: 10.1016/j.neuro.2025.05.010. Epub 2025 May 31. Neurotoxicology. 2025. PMID: 40456492 Review.
References
-
- Algarroba G. N., Rekawek P., Vahanian S. A., Khullar P., Palaia T., Peltier M. R., et al. (2020). Visualization of Severe Acute Respiratory Syndrome Coronavirus 2 Invading the Human Placenta Using Electron Microscopy. Am. J. Obstet. Gynecol. 223 (2), 275–278. 10.1016/j.ajog.2020.05.023 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

