Re-evaluating expanding intravenous catheters in medical practice
- PMID: 34250270
- PMCID: PMC8247936
- DOI: 10.1002/hsr2.318
Re-evaluating expanding intravenous catheters in medical practice
Abstract
Background: Intravenous catheters are common and essential devices within medical practice. Their placement can be difficult, leading to application of several technologies to improve success. Functionally expanding catheters were once an exciting technology, derailed clinically by hypersensitivity reactions. The exact cause of reactions, attributed to Aquavene catheter materials, remains unknown.
Aims: To reinvestigate functionally expanding intravenous catheters.
Materials and methods: The history of the functionally expanding intravenous catheter is presented here along with its utility in current medical practice, potential for further investigation, and possible redesign of these once promising devices.
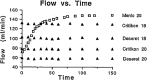
Results: This review demonstrates clinical utility and a lack of definitive cause for failure of the previous functionally expanding intravenous catheter design. As Aquavene materials themselves are commonly considered the cause of hypersensitivity reactions which removed expanding intravenous catheters from the market, this review found several possible substitutes for this material for use in any redesign.
Discussion and conclusion: The functionally expanding intravenous catheter failed due to hypersensitivity reactions in patients. Alternative materials exist for a possible redesign on this once promising clinical product.
Keywords: Aquavene; UV cross‐linking; anaphylactoid reaction; biopolymer; heterogeneous network; intravenous catheter; poly(ethylene glycol).
© 2021 The Authors. Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Marsh N, Webster J, Larson E, Cooke M, Mihala G, Rickard CM. Observational study of peripheral intravenous catheter outcomes in adult hospitalized patients: a multivariable analysis of peripheral intravenous catheter failure. J Hosp Med. 2017;13(2):83‐89. - PubMed
-
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs. 2015;38(3):189‐203. - PubMed
-
- Alexandrou E, Ray‐Barruel G, Carr PJ, et al. Use of short peripheral intravenous catheters: characteristics, management, and outcomes worldwide. J Hosp Med. 2018;13(5):E1‐E7. - PubMed
-
- Jones BR, Scheller MS. Flow increases with an enlarging intravenous catheter. J Clin Anesth. 1992;4(2):120‐122. - PubMed
-
- Cooke JE, Walker JM. Evaluation of a unique hydratable i.v. catheter. Anesthesiology. 1987;67(supplement):A208.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials