Plasma ctDNA Response Is an Early Marker of Treatment Effect in Advanced NSCLC
- PMID: 34250387
- PMCID: PMC8232122
- DOI: 10.1200/PO.20.00419
Plasma ctDNA Response Is an Early Marker of Treatment Effect in Advanced NSCLC
Abstract
Plasma circulating tumor DNA (ctDNA) analysis is routine for genotyping of advanced non-small-cell lung cancer (NSCLC); however, early response assessment using plasma ctDNA has yet to be well characterized.
Materials and methods: Patients with advanced EGFR-mutant NSCLC across three phase I NCI osimertinib combination trials were analyzed in this study, and an institutional cohort of patients with KRAS-, EGFR-, and BRAF-mutant advanced NSCLC receiving systemic treatment was used for validation. Plasma was collected before treatment initiation and serially before each cycle of therapy, and key driver mutations in ctDNA were characterized by droplet digital polymerase chain reaction. Timing of plasma versus imaging response was compared in a separate cohort of patients with EGFR-mutant NSCLC treated with osimertinib. Across cohorts, we also studied ctDNA variability before treatment start.
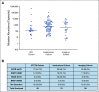
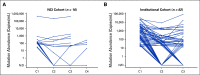
Results: In the NCI cohort, 14/16 (87.5%) patients exhibited ≥ 90% decrease in mutation abundance by the first on-treatment timepoint (20-28 days from treatment start) with minimal subsequent change. Similarly, 47/56 (83.9%) patients with any decrease in the institutional cohort demonstrated ≥ 90% decrease in mutation abundance by the first follow-up draw (7-30 days from treatment start). All 16 patients in the imaging cohort with radiographic partial response showed best plasma response within one cycle, preceding best radiographic response by a median of 24 weeks (range: 3-147 weeks). Variability in ctDNA levels before treatment start was observed.
Conclusion: Plasma ctDNA response is an early phenomenon, with the majority of change detectable within the first cycle of therapy. These kinetics may offer an opportunity for early insight into treatment effect before standard imaging timepoints.
© 2021 by American Society of Clinical Oncology.
Figures



References
-
- Leighl NB, Page RD, Raymond VM, et al. : Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non–small cell lung cancer. Clin Cancer Res 25:4691-4700, 2019 - PubMed
-
- Abbosh C, Birkbak NJ, Swanton C: Early stage NSCLC—challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol 15:577-586, 2018 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

