A Systematic and Critical Review of Discrete Choice Experiments in Asthma and Chronic Obstructive Pulmonary Disease
- PMID: 34250574
- PMCID: PMC8738458
- DOI: 10.1007/s40271-021-00536-w
A Systematic and Critical Review of Discrete Choice Experiments in Asthma and Chronic Obstructive Pulmonary Disease
Erratum in
-
Correction to: A Systematic and Critical Review of Discrete Choice Experiments in Asthma and Chronic Obstructive Pulmonary Disease.Patient. 2022 Jan;15(1):145. doi: 10.1007/s40271-021-00545-9. Patient. 2022. PMID: 34423399 Free PMC article. No abstract available.
Abstract
Background: Regulators have called for greater emphasis on the role of the patient voice to inform medical product development and decision making, and expert guidelines and reports for asthma and chronic obstructive pulmonary disease (COPD) both explicitly recommend the consideration of patient preferences in the management of these diseases. Discrete choice experiments (DCEs) are commonly used to quantify stakeholders' treatment preferences and estimate the trade-offs they are willing to make between outcomes such as treatment benefits and risks.
Objective: The aim of this systematic literature review is to provide an up-to-date and critical review of DCEs published in asthma and COPD; specifically, we aim to evaluate the subject of preference studies conducted in asthma and COPD, what attributes have been included, stakeholders' preferences, and the consistency in reporting of instrument development, testing and reporting of results.
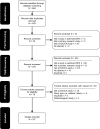
Methods: A systematic review of published DCEs on asthma and COPD treatments was conducted using Embase, Medline and the Cochrane Database of Systematic Reviews. Studies were included if they included a DCE conducted in a relevant population (e.g. patients with asthma or COPD or their caregivers, asthma or COPD-treating clinicians, or the general population), and reported quantitative outcomes on participants' preferences. Study characteristics were summarised descriptively, and descriptive analyses of attribute categories, consistency in reporting on key criteria, and stakeholder preferences were undertaken.
Results: A total of 33 eligible studies were identified, including 28 unique DCEs. The majority (n = 20; 71%) of studies were conducted in a patient sample. Studies focused on inhaler treatments, and included attributes in five key categories: symptoms and treatment benefits (n = 23; 82%), treatment convenience (n = 19; 68%), treatment cost (n = 17; 61%), treatment risks (n = 13; 46%), and other (n = 10; 36%). Symptoms and treatment benefits were the attributes most frequently ranked as important to patients (n = 26, 72%), followed by treatment risks (n = 7, 39%). Several studies (n = 9, 32%) did not qualitatively pre-test their DCE, and a majority did not report the uncertainty in estimated outcomes (n = 18; 64%).
Conclusions: DCEs in asthma and COPD have focused on treatment benefits and convenience, with less evidence generated on participants' risk tolerance. Quality criteria and reporting standards are needed to promote study quality and ensure consistency in reporting between studies.
© 2021. The Author(s).
Conflict of interest statement
Hannah Collacott, Dian Zhang, Sebastian Heidenreich, and Tommi Tervonen are employees of Evidera, which provides consulting and other research services to pharmaceutical, medical device, and related organisations. In their salaried positions, they work with a variety of companies and organisations and are precluded from receiving payment or honoraria directly from these organisations for services rendered.
Figures
References
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2021 update). 2021. https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V.... Accessed 3 Jun 2021.
-
- Global Initiative for Chronic Obstructive Lung Disease Inc. Pocket Guide to COPD: Diagnosis, Management, and Prevention. 2020. https://goldcopd.org/wp-content/uploads/2020/03/GOLD-2020-POCKET-GUIDE-v.... Accessed 17 Dec 2020.
-
- European Medicines Agency (EMA). EMA Regulatory Science to 2025: Strategic reflection. 2020. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/e.... Accessed 20 May 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

