Enrollment with and without exception from informed consent in a pilot trial of tranexamic acid in children with hemorrhagic injuries
- PMID: 34250690
- PMCID: PMC8712344
- DOI: 10.1111/acem.14343
Enrollment with and without exception from informed consent in a pilot trial of tranexamic acid in children with hemorrhagic injuries
Abstract
Background: Federal exception from informed consent (EFIC) procedures allow studies to enroll patients with time-sensitive, life-threatening conditions when written consent is not feasible. Our objective was to compare enrollment rates with and without EFIC in a trial of tranexamic acid (TXA) for children with hemorrhagic injuries.
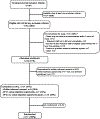
Methods: We conducted a four-center randomized controlled pilot and feasibility trial evaluating TXA in children with severe hemorrhagic brain and/or torso injuries. We initiated the trial enrolling patients without EFIC. After 3 months of enrollment, we met our a priori futility threshold and paused the trial to incorporate EFIC procedures and obtain regulatory approval. We then restarted the trial allowing EFIC if the guardian was unable to provide timely written consent. We used descriptive statistics to compare characteristics of eligible patients approached with and without EFIC procedures. We also calculated the time delay to restart the trial using EFIC.
Results: We enrolled one of 15 (6.7%) eligible patients (0.17 per site per month) prior to using EFIC procedures. Of the 14 missed eligible patients, seven (50%) were not enrolled because guardians were not present or were injured and unable to provide written consent. After obtaining approval for EFIC, we enrolled 30 of 48 (62.5%) eligible patients (1.34 per site per month). Of these 30 patients, 22 (73.3%) were enrolled with EFIC. Of the 22, no guardians refused written consent after randomization. There were no significant differences in the eligibility rate and patient characteristics enrolled with and without EFIC procedures. Across all sites, the mean delay to restart the trial using EFIC procedures was 12 months.
Conclusions: In a multicenter trial of severely injured children, the use of EFIC procedures greatly increased the enrollment rate and was well accepted by guardians. Initiating the trial without EFIC procedures led to a significant delay in enrollment.
Keywords: exception from informed consent (EFIC); pediatric trauma; tranexamic acid.
© 2021 by the Society for Academic Emergency Medicine.
Conflict of interest statement
Conflict of Interest Disclosure:
All listed authors have no conflicts of interest to disclose.
Figures


References
-
- Exception from Informed Consent Requirements for Emergency Research: Guidance for Institutional Review Boards, Clinical Investigators, and Sponsors. Federal Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed February 25, 2021.
-
- Govindarajan P, Dickert NW, Meeker M, et al. Emergency research: using exception from informed consent, evaluation of community consultations. Acad Emerg Med 2013;20:98–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

