Piezoelectric Nanomaterials Activated by Ultrasound: The Pathway from Discovery to Future Clinical Adoption
- PMID: 34251189
- PMCID: PMC8397402
- DOI: 10.1021/acsnano.1c03087
Piezoelectric Nanomaterials Activated by Ultrasound: The Pathway from Discovery to Future Clinical Adoption
Abstract
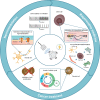
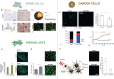
Electrical stimulation has shown great promise in biomedical applications, such as regenerative medicine, neuromodulation, and cancer treatment. Yet, the use of electrical end effectors such as electrodes requires connectors and batteries, which dramatically hamper the translation of electrical stimulation technologies in several scenarios. Piezoelectric nanomaterials can overcome the limitations of current electrical stimulation procedures as they can be wirelessly activated by external energy sources such as ultrasound. Wireless electrical stimulation mediated by piezoelectric nanoarchitectures constitutes an innovative paradigm enabling the induction of electrical cues within the body in a localized, wireless, and minimally invasive fashion. In this review, we highlight the fundamental mechanisms of acoustically mediated piezoelectric stimulation and its applications in the biomedical area. Yet, the adoption of this technology in a clinical practice is in its infancy, as several open issues, such as piezoelectric properties measurement, control of the ultrasound dose in vitro, modeling and measurement of the piezo effects, knowledge on the triggered bioeffects, therapy targeting, biocompatibility studies, and control of the ultrasound dose delivered in vivo, must be addressed. This article explores the current open challenges in piezoelectric stimulation and proposes strategies that may guide future research efforts in this field toward the translation of this technology to the clinical scene.
Keywords: cancer treatment; electric stimuli; mechanoelectrical transduction; neuromodulation; piezoelectric effect; piezoelectric nanomaterials; regenerative medicine; ultrasound.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Escoffre J.-M., Bouakaz A., Eds. Therapeutic Ultrasound; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Vol. 880.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

