PET Imaging of Translocator Protein as a Marker of Malaria-Associated Lung Inflammation
- PMID: 34251290
- PMCID: PMC8445173
- DOI: 10.1128/IAI.00024-21
PET Imaging of Translocator Protein as a Marker of Malaria-Associated Lung Inflammation
Abstract
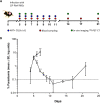
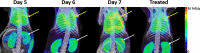
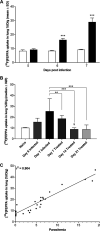
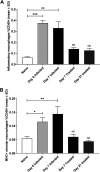
Malaria-associated acute respiratory distress syndrome (MA-ARDS) is a severe complication of malaria that occurs despite effective antimalarial treatment. Currently, noninvasive imaging procedures such as chest X-rays are used to assess edema in established MA-ARDS, but earlier detection methods are needed to reduce morbidity and mortality. The early stages of MA-ARDS are characterized by the infiltration of leukocytes, in particular monocytes/macrophages; thus, monitoring of immune infiltrates may provide a useful indicator of early pathology. In this study, Plasmodium berghei ANKA-infected C57BL/6 mice, a rodent model of MA-ARDS, were longitudinally imaged using the 18-kDa translocator protein (TSPO) imaging agent [18F]FEPPA as a marker of macrophage accumulation during the development of pathology and in response to combined artesunate and chloroquine diphosphate (ART+CQ) therapy. [18F]FEPPA uptake was compared to blood parasitemia levels and to levels of pulmonary immune cell infiltrates by using flow cytometry. Infected animals showed rapid increases in lung retention of [18F]FEPPA, correlating well with increases in blood parasitemia and pulmonary accumulation of interstitial inflammatory macrophages and major histocompatibility complex class II (MHC-II)-positive alveolar macrophages. Treatment with ART+CQ abrogated this increase in parasitemia and significantly reduced both lung uptake of [18F]FEPPA and levels of macrophage infiltrates. We conclude that retention of [18F]FEPPA in the lungs is well correlated with changes in blood parasitemia and levels of lung-associated macrophages during disease progression and in response to ART+CQ therapy. With further development, TSPO biomarkers may have the potential to accurately assess the early onset of MA-ARDS.
Keywords: PET; PbA; lung; macrophages; malaria.
Figures
References
-
- WHO Global Malaria Programme. 30November2020. World malaria report 2020. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

