Estimated SARS-CoV-2 Seroprevalence in US Patients Receiving Dialysis 1 Year After the Beginning of the COVID-19 Pandemic
- PMID: 34251441
- PMCID: PMC8276082
- DOI: 10.1001/jamanetworkopen.2021.16572
Estimated SARS-CoV-2 Seroprevalence in US Patients Receiving Dialysis 1 Year After the Beginning of the COVID-19 Pandemic
Abstract
Importance: Seroprevalence studies complement data on detected cases and attributed deaths in assessing the cumulative spread of the SARS-CoV-2 virus.
Objective: To estimate seroprevalence of SARS-CoV-2 antibodies in patients receiving dialysis and adults in the US in January 2021 before the widespread introduction of COVID-19 vaccines.
Design, setting, and participants: This cross-sectional study used data from the third largest US dialysis organization (US Renal Care), which has facilities located nationwide, to estimate SARS-CoV-2 seroprevalence among US patients receiving dialysis. Remainder plasma (ie, plasma that would have otherwise been discarded) of all patients receiving dialysis at US Renal Care facilities from January 1 to 31, 2021, was tested for SARS-CoV-2 antibodies. Patients were excluded if they had a documented dose of SARS-CoV-2 vaccination or if a residence zip code was missing from electronic medical records. Crude seroprevalence estimates from this sample (January 2021) were standardized to the US adult population using the 2018 American Community Survey 1-year estimates and stratified by age group, sex, self-reported race/ethnicity, neighborhood race/ethnicity composition, neighborhood income level, and urban or rural status. These data and case detection rates were then compared with data from a July 2020 subsample of patients who received dialysis at the same facilities.
Exposures: Age, sex, race/ethnicity, and region of residence as well as neighborhood race/ethnicity composition, poverty, population density, and urban or rural status.
Main outcomes and measures: The spike protein receptor-binding domain total antibody assay (Siemens Healthineers; manufacturer-reported sensitivity of 100% and specificity of 99.8%) was used to estimate crude SARS-CoV-2 seroprevalence in the unweighted sample, and then the estimated seroprevalence rates for the US dialysis and adult populations were calculated, adjusting for age, sex, and region.
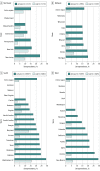
Results: A total of 21 464 patients (mean [SD] age, 63.1 [14.2] years; 12 265 men [57%]) were included in the unweighted sample from January 2021. The patients were disproportionately older (aged 65-79 years, 7847 [37%]; aged ≥80 years, 2668 [12%]) and members of racial/ethnic minority groups (Hispanic patients, 2945 [18%]; non-Hispanic Black patients, 4875 [29%]). Seroprevalence of SARS-CoV-2 antibodies was 18.9% (95% CI, 18.3%-19.5%) in the sample, with a seroprevalence of 18.7% (95% CI, 18.1%-19.2%) standardized to the US dialysis population, and 21.3% (95% CI, 20.3%-22.3%) standardized to the US adult population. In the unweighted sample, younger persons (aged 18-44 years, 25.9%; 95% CI, 24.1%-27.8%), those who self-identified as Hispanic or living in Hispanic neighborhoods (25.1%; 95% CI, 23.6%-26.4%), and those living in the lowest-income neighborhoods (24.8%; 95% CI, 23.2%-26.5%) were among the subgroups with the highest seroprevalence. Little variability was observed in seroprevalence by geographic region, population density, and urban or rural status in the January 2021 sample (largest regional difference, 1.2 [95% CI, 1.1-1.3] higher odds of seroprevalence in residents of the Northeast vs West).
Conclusions and relevance: In this cross-sectional study of patients receiving dialysis in the US, fewer than 1 in 4 patients had evidence of SARS-CoV-2 antibodies 1 year after the first case of SARS-CoV-2 infection was detected in the US. Results standardized to the US population indicate similar prevalence of antibodies among US adults. Vaccine introduction to younger individuals, those living in neighborhoods with a large population of racial/ethnic minority residents, and those living in low-income neighborhoods may be critical to disrupting the spread of infection.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention . CDC COVID data tracker. Accessed January 24, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

