Evaluation of the Effects of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19 : A Randomized Trial
- PMID: 34251903
- PMCID: PMC8279143
- DOI: 10.7326/M21-0653
Evaluation of the Effects of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19 : A Randomized Trial
Abstract
Background: New treatment modalities are urgently needed for patients with COVID-19. The World Health Organization (WHO) Solidarity trial showed no effect of remdesivir or hydroxychloroquine (HCQ) on mortality, but the antiviral effects of these drugs are not known.
Objective: To evaluate the effects of remdesivir and HCQ on all-cause, in-hospital mortality; the degree of respiratory failure and inflammation; and viral clearance in the oropharynx.
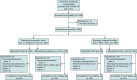
Design: NOR-Solidarity is an independent, add-on, randomized controlled trial to the WHO Solidarity trial that included biobanking and 3 months of clinical follow-up (ClinicalTrials.gov: NCT04321616).
Setting: 23 hospitals in Norway.
Patients: Eligible patients were adults hospitalized with confirmed SARS-CoV-2 infection.
Intervention: Between 28 March and 4 October 2020, a total of 185 patients were randomly assigned and 181 were included in the full analysis set. Patients received remdesivir (n = 42), HCQ (n = 52), or standard of care (SoC) (n = 87).
Measurements: In addition to the primary end point of WHO Solidarity, study-specific outcomes were viral clearance in oropharyngeal specimens, the degree of respiratory failure, and inflammatory variables.
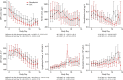
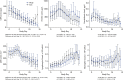
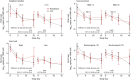
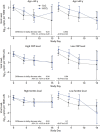
Results: No significant differences were seen between treatment groups in mortality during hospitalization. There was a marked decrease in SARS-CoV-2 load in the oropharynx during the first week overall, with similar decreases and 10-day viral loads among the remdesivir, HCQ, and SoC groups. Remdesivir and HCQ did not affect the degree of respiratory failure or inflammatory variables in plasma or serum. The lack of antiviral effect was not associated with symptom duration, level of viral load, degree of inflammation, or presence of antibodies against SARS-CoV-2 at hospital admittance.
Limitation: The trial had no placebo group.
Conclusion: Neither remdesivir nor HCQ affected viral clearance in hospitalized patients with COVID-19.
Primary funding source: National Clinical Therapy Research in the Specialist Health Services, Norway.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
