Development and multimodal characterization of an elastase-induced emphysema mouse disease model for the COPD frequent bacterial exacerbator phenotype
- PMID: 34252004
- PMCID: PMC8276669
- DOI: 10.1080/21505594.2021.1937883
Development and multimodal characterization of an elastase-induced emphysema mouse disease model for the COPD frequent bacterial exacerbator phenotype
Abstract
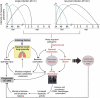
Chronic obstructive pulmonary disease (COPD) patients undergo infectious exacerbations whose frequency identifies a clinically meaningful phenotype. Mouse models have been mostly used to separately study both COPD and the infectious processes, but a reliable model of the COPD frequent exacerbator phenotype is still lacking. Accordingly, we first established a model of single bacterial exacerbation by nontypeable Haemophilus influenzae (NTHi) infection on mice with emphysema-like lesions. We characterized this single exacerbation model combining both noninvasive in vivo imaging and ex vivo techniques, obtaining longitudinal information about bacterial load and the extent of the developing lesions and host responses. Bacterial load disappeared 48 hours post-infection (hpi). However, lung recovery, measured using tests of pulmonary function and the disappearance of lung inflammation as revealed by micro-computed X-ray tomography, was delayed until 3 weeks post-infection (wpi). Then, to emulate the frequent exacerbator phenotype, we performed two recurrent episodes of NTHi infection on the emphysematous murine lung. Consistent with the amplified infectious insult, bacterial load reduction was now observed 96 hpi, and lung function recovery and disappearance of lesions on anatomical lung images did not happen until 12 wpi. Finally, as a proof of principle of the use of the model, we showed that azithromycin successfully cleared the recurrent infection, confirming this macrolide utility to ameliorate infectious exacerbation. In conclusion, we present a mouse model of recurrent bacterial infection of the emphysematous lung, aimed to facilitate investigating the COPD frequent exacerbator phenotype by providing complementary, dynamic information of both infectious and inflammatory processes.
Keywords: Lung emphysema; bacterial exacerbation; inflammation; micro-CT; test of pulmonary function.
Figures





References
-
- Celli BR, Wedzicha JA.. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1257–1266. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31553837 - PubMed
-
- Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17765528 - PMC - PubMed
-
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. Available from. https://www.ncbi.nlm.nih.gov/pubmed/20843247 - PubMed
-
- Lopez-Campos JL, Miravitlles M, De La Rosa Carrillo D, et al. Current challenges in chronic bronchial infection in patients with chronic obstructive pulmonary disease. J Clin Med. 2020;9(6):1639. Available from: https://www.ncbi.nlm.nih.gov/pubmed/32481769 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
