Mitochondrial Ca2+ homeostasis in trypanosomes
- PMID: 34253297
- PMCID: PMC10424509
- DOI: 10.1016/bs.ircmb.2021.01.002
Mitochondrial Ca2+ homeostasis in trypanosomes
Abstract
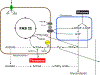
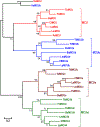
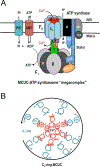
Mitochondrial calcium ion (Ca2+) uptake is important for buffering cytosolic Ca2+ levels, for regulating cell bioenergetics, and for cell death and autophagy. Ca2+ uptake is mediated by a mitochondrial Ca2+ uniporter (MCU) and the discovery of this channel in trypanosomes has been critical for the identification of the molecular nature of the channel in all eukaryotes. However, the trypanosome uniporter, which has been studied in detail in Trypanosoma cruzi, the agent of Chagas disease, and T. brucei, the agent of human and animal African trypanosomiasis, has lineage-specific adaptations which include the lack of some homologues to mammalian subunits, and the presence of unique subunits. Here, we review newly emerging insights into the role of mitochondrial Ca2+ homeostasis in trypanosomes, the composition of the uniporter, its functional characterization, and its role in general physiology.
Keywords: Acidocalcisome; Calcium; Cell bioenergetics; Inositol phosphate; Mitochondria; Mitochondrial calcium uniporter; Polyphosphate; Trypanosomatids.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures

References
-
- Adl SM, Simpson AG, Lane CE, Lukes J, Bass D, Bowser SS, Brown MW, Burki F, Dunthorn M, Hampl V, Heiss A, Hoppenrath M, Lara E, Le Gall L, Lynn DH, McManus H, Mitchell EA, Mozley-Stanridge SE, Parfrey LW, Pawlowski J, Rueckert S, Shadwick L, Schoch CL, Smirnov A,Spiegel FW, 2012. The revised classification of eukaryotes. J Eukaryot Microbiol 59, 429–493. - PMC - PubMed
-
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr.,Jonas EA, 2014. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111, 10580–10585. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

