Uptake of co-testing with HPV and cytology for cervical screening: A population-based evaluation in the United States
- PMID: 34253387
- PMCID: PMC8405560
- DOI: 10.1016/j.ygyno.2021.06.029
Uptake of co-testing with HPV and cytology for cervical screening: A population-based evaluation in the United States
Abstract
Objectives: Human papillomavirus (HPV) testing for cervical screening has been shown to increase the yield of precancerous disease and reduce the incidence of cervical cancer more than cytology alone. Here we document the state-wide uptake of co-testing with HPV and cytology in women aged 30-64 years as recommended by national and international bodies.
Methods: Registry-based study of all screening cytology and HPV tests in New Mexico from 2008 to 2019 among women aged 21-64 years, with a focus on cytology negative tests to distinguish co-testing from reflex HPV testing to triage equivocal or mildly abnormal cytology.
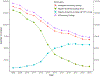
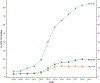
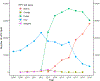
Results: A total of 1,704,055 cervical screening tests from 681,440 women aged 21-64 years in the state of New Mexico were identified. The proportion of screening tests which were co-tests rose from 5.6% in 2008 to 84.3% in 2019 among women aged 30-64 years with a marked change from the near exclusive use of the Hybrid Capture II HPV test, (a signal amplified test method) to the use of target amplified HPV tests. The largest increases were seen between 2013 and 2015, reflecting the introduction and adoption of new clinical guidelines. Increases in co-testing were also seen in younger women.
Conclusions: Co-testing is now well established in women aged 30-64 years, but smaller increases have also been seen at younger ages, although this is not currently recommended. The impact of co-testing on cervical disease outcomes and number of colposcopies and biopsies in routine population settings remain important, especially in young women.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest JC and CMW have received funds from grants, cooperative agreements or subcontracts related to cervical screening and triage through their respective institutions. JC reports personal fees from Hologic and grants from Becton Dickinson (BD), Qiagen and Gene First all outside the submitted work. CMW reports receiving reagents and equipment from Roche Molecular Systems, Roche/Ventana Medical Systems, Hologic and Genera Biosystems, research funding from Hologic all through her institution and outside of the submitted work, personal fees from BD. All other authors report no potential conflicts of interest.
Figures
References
-
- Saslow D, Runowicz CD, Solomon D, et al.American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002;52(6):342–62. - PubMed
-
- Schiffman M, Herrero R, Hildesheim A, et al.HPV DNA testing in cervical cancer screening: results from women in a high-risk province of Costa Rica. Jama 2000;283(1):87–93. - PubMed
-
- Cuzick J, Clavel C, Petry KU, et al.Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006;119(5):1095–101. - PubMed
-
- Bulkmans NW, Berkhof J, Rozendaal L, et al.Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007;370(9601):1764–72. - PubMed
-
- Ronco G, Dillner J, Elfström KM, et al.Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014;383(9916):524–32. - PubMed

